Hydrogen is an unusual element because it behaves in some ways like the alkali metal elements and in other ways like nonmetals. Its properties can be explained in part by its electron configuration and by the values for its ionization energy and electron affinity. (a) Explain why the electron affinity of hydrogen is much closer to the values for the alkali elements than for the halogens.

Which of the following is the expected product of the reaction of K(s) and H2(g)? (i) KH(s), (ii) K2H(s), (iii) KH2(s), (iv) K2H2(s), or (v) K(s) and H2(g) will not react with one another.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Reactivity of Alkali Metals

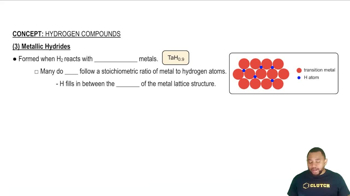
Formation of Metal Hydrides
Stoichiometry of Chemical Reactions

The first ionization energy of the oxygen molecule is the energy required for the following process: O21g2¡O2 +1g2 + e- The energy needed for this process is 1175 kJ>mol, very similar to the first ionization energy of Xe. Would you expect O2 to react with F2? If so, suggest a product or products of this reaction.
It is possible to define metallic character as we do in this book and base it on the reactivity of the element and the ease with which it loses electrons. Alternatively, one could measure how well electricity is conducted by each of the elements to determine how 'metallic' the elements are. On the basis of conductivity, there is not much of a trend in the periodic table: Silver is the most conductive metal, and manganese the least. Look up the first ionization energies of silver and manganese; which of these two elements would you call more metallic based on the way we define it in this book?
