Identify each statement as true or false: (a) Cations are larger than their corresponding neutral atoms.
Which neutral atom is isoelectronic with each of the following ions? Ga3+, Zr4+, Mn7+, I−, Pb2+.

Verified Solution
Key Concepts
Isoelectronic Species
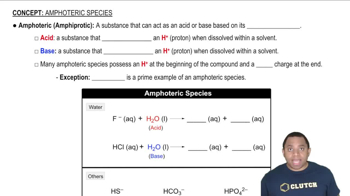
Electron Configuration

Charge and Electron Count

Identify each statement as true or false: (b) Li+ is smaller than Li.
Use data from Appendix C, Figure 7.10, and Figure 7.12 to calculate the lattice energy of RbCl.
Some ions do not have a corresponding neutral atom that has the same electron configuration. For each of the following ions, identify the neutral atom that has the same number of electrons and determine if this atom has the same electron configuration. (a) CI−, (b) Sc3+, (c) Fe2+, (d) Zn2+, (e) Sn4+.
Consider the isoelectronic ions F- and Na+. (b) Using Equation 7.1 and assuming that core electrons contribute 1.00 and valence electrons contribute 0.00 to the screening constant, S, calculate Zeff for the 2p electrons in both ions.
Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
