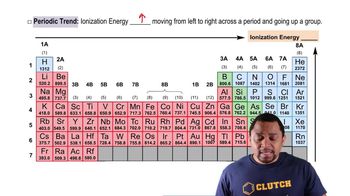
Textbook Question
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 201 pm (Li–F), 282 pm (Na–Cl), 330 pm (K–Br), and 367 pm (Rb–I), respectively. (c) What estimates of the cation– anion distance would you obtain for these four compounds using neutral atom bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
1349
views