Use bond enthalpies in Table 5.4 to estimate H for each of the following reactions: (a)
(b) A particular chip snack food is composed of 12% protein, 14% fat, and the rest carbohydrate. What percentage of the calorie content of this food is fat?

Verified Solution
Key Concepts
Caloric Content of Macronutrients
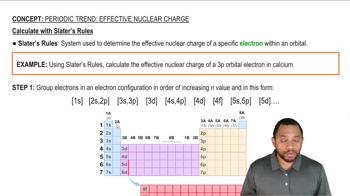
Percentage Composition

Total Caloric Calculation

(a) The nitrogen atoms in an N2 molecule are held together by a triple bond; use enthalpies of formation in Appendix C to estimate the enthalpy of this bond, D(N‚N). (b) Consider the reaction between hydrazine and hydrogen to produce ammonia, N2H41g2 + H21g2¡2 NH31g2. Use enthalpies of formation and bond enthalpies to estimate the enthalpy of the nitrogen– nitrogen bond in N2H4. (c) Based on your answers to parts (a) and (b), would you predict that the nitrogen–nitrogen bond in hydrazine is weaker than, similar to, or stronger than the bond in N2 ?
Consider the reaction 2 H2(g) + O2(g) → 2 H2O(l). (a) Use the bond enthalpies in Table 5.4 to estimate H for this reaction, ignoring the fact that water is in the liquid state.
(a) A serving of a particular ready-to-serve brown & wild rice meal contains 4.5 g fat, 42 g carbohydrate, and 4.0 g protein. Estimate the number of calories in a serving.
The heat of combustion of fructose, C6H12O6, is -2812 kJ/mol. If a fresh golden delicious apple weighing 120 g contains 16.0 g of fructose, what caloric content does the fructose contribute to the apple?
The heat of combustion of ethanol, C2H5OH(l), is -1367 kJ/mol. A bottle of stout (dark beer) contains up to 6.0% ethanol by mass. Assuming the density of the beer to be 1.0 g/mL, what is the caloric content due to the alcohol (ethanol) in a bottle of beer (500 mL)?
