We have learned in this chapter that many ionic solids dissolve in water as strong electrolytes; that is, as separated ions in solution. Which statement is most correct about this process? (a) Water is a strong acid and therefore is good at dissolving ionic solids. (b) Water is good at solvating ions because the hydrogen and oxygen atoms in water molecules bear partial charges. (c) The hydrogen and oxygen bonds of water are easily broken by ionic solids.
Ch.4 - Reactions in Aqueous Solution
Chapter 4, Problem 17c
Ignoring protolysis reactions (i.e. proton transfer reaction), specify what ions are present in a solution upon dissolving each of the following substances in water: (c) Na2Cr2O7
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical formula of the compound: Na_2Cr_2O_7.
Recognize that Na_2Cr_2O_7 is an ionic compound composed of sodium ions (Na^+) and dichromate ions (Cr_2O_7^{2-}).
When Na_2Cr_2O_7 dissolves in water, it dissociates into its constituent ions.
Write the dissociation equation: Na_2Cr_2O_7 (s) → 2 Na^+ (aq) + Cr_2O_7^{2-} (aq).
Conclude that the ions present in the solution are Na^+ and Cr_2O_7^{2-}.

Verified Solution
Video duration:
53sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dissociation of Ionic Compounds
When ionic compounds like Na2Cr2O7 dissolve in water, they dissociate into their constituent ions. This process involves the separation of the positive and negative ions due to the interaction with water molecules, which stabilizes the ions in solution.
Recommended video:
Guided course

Ionic Compounds Naming
Ionic Species in Solution
In the case of Na2Cr2O7, the compound dissociates into sodium ions (Na+) and dichromate ions (Cr2O7^2-). Understanding the specific ions formed is crucial for predicting the chemical behavior of the solution, including its reactivity and potential applications.
Recommended video:
Guided course
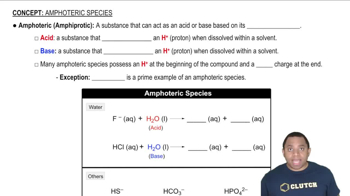
Amphoteric Species
Role of Water as a Solvent
Water is often referred to as a 'universal solvent' due to its ability to dissolve many ionic and polar substances. The polar nature of water molecules allows them to surround and solvate ions, facilitating their movement and interaction in solution, which is essential for various chemical processes.
Recommended video:
Guided course

Reaction with Water
Related Practice
Textbook Question
950
views
Textbook Question
Would you expect that an anion would be physically closer to the oxygen or to the hydrogens of water molecules that surround it in solution?
406
views
Textbook Question
Ignoring protolysis reactions (i.e. proton transfer reaction), specify what ions are present in a solution upon dissolving each of the following substances in water: (a)Li2CO3
376
views
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (a) MgI2
462
views
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (b) K2CO3
886
views
1
comments
Textbook Question
Specify what ions are present upon dissolving each of the following substances in water: (c) HClO4
543
views
