Indicate the concentration of each ion or molecule present in the following solutions: (a) 0.35 M K3PO4 (b) 1.3×10−2 M MgSO4 (c) 0.0184 M CH3CH2OH

Indicate the concentration of each ion present in the solution formed by mixing: (b) 44.0 mL of 0.100 M Na2SO4 with 25.0 mL of 0.150 M KCl
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Molarity

Stoichiometry

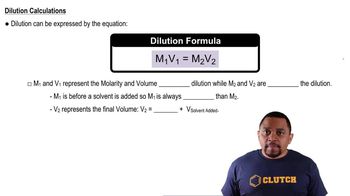
Dilution
Ignoring protolysis reactions, indicate the concentration of each ion or molecule present in the following solutions: (d) a mixture of 45.0 mL of 0.272 M NaCl and 65.0 mL of 0.0247 M (NH4)2CO3. Assume that the volumes are additive.
Indicate the concentration of each ion present in the solution formed by mixing: (a) 42.0 mL of 0.170 M NaOH with 37.6 mL of 0.400 M NaOH.
Indicate the concentration of each ion present in the solution formed by mixing: (c) 3.60 g KCl in 75.0 mL of 0.250 M CaCl2 solution. Assume that the volumes are additive.
(a) You have a stock solution of 14.8 M NH3. How many milliliters of this solution should you dilute to make 1000.0 mL of 0.250 M NH3?
(b) If you take a 10.0-mL portion of the stock solution and dilute it to a total volume of 0.500 L, what will be the concentration of the final solution?
