Indicate the concentration of each ion present in the solution formed by mixing: (c) 3.60 g KCl in 75.0 mL of 0.250 M CaCl2 solution. Assume that the volumes are additive.
(b) If you dilute 10.0 mL of the stock solution to a final volume of 0.250 L, what will be the concentration of the diluted solution?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Dilution
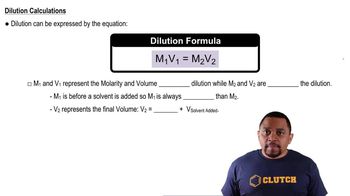
Concentration

Volume

(b) If you take a 10.0-mL portion of the stock solution and dilute it to a total volume of 0.500 L, what will be the concentration of the final solution?
(a) How many milliliters of a stock solution of 6.0 M HNO3 would you have to use to prepare 110 mL of 0.500 M HNO3?
medical lab is testing a new anticancer drug on cancer cells. The drug stock solution concentration is 1.5 * 10-9 M, and 1.00 mL of this solution will be delivered to a dish containing 2.0 * 105 cancer cells in 5.00 mL of aqueous fluid. What is the ratio of drug molecules to the number of cancer cells in the dish?
Calicheamicin gamma-1, C55H74IN3O21S4, is one of the most potent antibiotics known: one molecule kills one bacterial cell. Describe how you would (carefully!) prepare 25.00 mL of an aqueous calicheamicin gamma-1 solution that could kill 1.0 * 108 bacteria, starting from a 5.00 * 10-9M stock solution of the antibiotic.
Pure acetic acid, known as glacial acetic acid, is a liquid with a density of 1.049 g/mL at 25 C. Calculate the molarity of a solution of acetic acid made by dissolving 20.00 mL of glacial acetic acid at 25 C in enough water to make 250.0 mL of solution.
