Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO21s2 react with each mole of CO21g2. (b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced?
Aqueous solutions of three different substances, AX, AY, and AZ, are represented by the three accompanying diagrams. Identify each substance as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. (a)
(b)
(c)

Verified Solution
Key Concepts
Electrolytes

Strong vs. Weak Electrolytes

Conductivity Testing
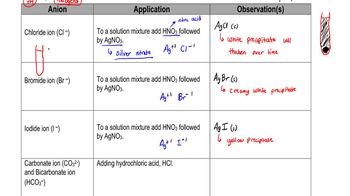
Acetone, CH3COCH3, is a nonelectrolyte; hypochlorous acid, HClO, is a weak electrolyte; and ammonium chloride, NH4Cl, is a strong electrolyte. (a) What are the solutes present in aqueous solutions of each compound? What solute particles are present in an aqueous solution of NH4Cl?
Which of the following schematic drawings best describes a solution of Li2SO4 in water (water molecules not shown for simplicity)?
Use the molecular representations shown here to classify each compound as a nonelectrolyte, a weak electrolyte, or a strong electrolyte (see Figure 4.6 for the element color scheme). (a)
The concept of chemical equilibrium is very important. Which one of the following statements is the most correct way to think about equilibrium? (a) If a system is at equilibrium, nothing is happening. (b) If a system is at equilibrium, the rate of the forward reaction is equal to the rate of the back reaction. (c) If a system is at equilibrium, the product concentration is changing over time.
You are presented with a white solid and told that due to careless labeling it is not clear if the substance is barium chloride, lead chloride, or zinc chloride. When you transfer the solid to a beaker and add water, the solid dissolves to give a clear solution. Next an Na2SO41aq2 solution is added and a white precipitate forms. What is the identity of the unknown white solid?
