The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2(79%) and O2(20%). In the cylinder of an automobile engine, nitrogen can react with oxygen to produce nitric oxide gas, NO. As NO is emitted from the tailpipe of the car, it can react with more oxygen to produce nitrogen dioxide gas. (c) The production of NOx gases is an unwanted side reaction of the main engine combustion process that turns octane, C8H18, into CO2 and water. If 85% of the oxygen in an engine is used to combust octane and the remainder used to produce nitrogen dioxide, calculate how many grams of nitrogen dioxide would be produced during the combustion of 500 g of octane.
One of the most bizarre reactions in chemistry is called the
Ugi reaction:
R1C(=O)R2 + R3 - NH2 + R4COOH + R5NC S
R4C(=O)N(R3)C(R1R2)C=ONHR5 + H2O

(a) Write out the balanced chemical equation for the Ugi reaction,
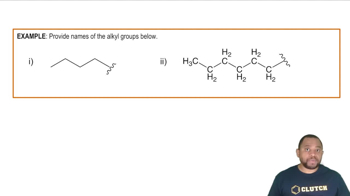
for the case where R = CH3CH2CH2CH2CH2CH2—
(this is called the hexyl group) for all compounds.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Ugi Reaction

Balanced Chemical Equation

Hexyl Group
The thermite reaction, Fe2O3 + Al → Al2O3 + Fe produces so much heat that the Fe product melts. This reaction is used industrially to weld metal parts under water, where a torch cannot be employed. It is also a favorite chemical demonstration in the lecture hall (on a small scale). (b) Calculate how many grams of aluminum are needed to completely react with 500.0 g of Fe2O3 in this reaction.
The thermite reaction, Fe2O3 + Al S Al2O3 + Fe produces so much heat that the Fe product melts. This reaction is used industrially to weld metal parts under water, where a torch cannot be employed. It is also a favorite chemical demonstration in the lecture hall (on a small scale). (d) If you performed the reverse reaction— aluminum oxide plus iron makes iron oxide plus aluminum—would that reaction have heat as a reactant or a product?
