Textbook Question
(b) Why are the amounts of products formed in a reaction determined only by the amount of the limiting reactant?
507
views

 Verified step by step guidance
Verified step by step guidance


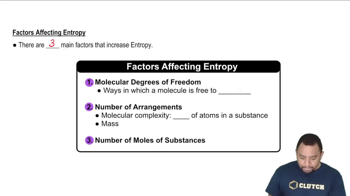
(b) Why are the amounts of products formed in a reaction determined only by the amount of the limiting reactant?
(c) Why should you base your choice of which compound is the limiting reactant on its number of initial moles, not on its initial mass in grams?
(a) Define the terms theoretical yield, actual yield, and percent yield.
(c) Can a reaction ever have 110% actual yield?
Consider the mixture of ethanol, C2H5OH, and O2 shown in the accompanying diagram. (b) Which reactant is the limiting reactant?
Consider the mixture of ethanol, C2H5OH, and O2 shown in the accompanying diagram. (c) How many molecules of CO2, H2O, C2H5OH, and O2 will be present if the reaction goes to completion?