Here are the essential concepts you must grasp in order to answer the question correctly.
Tetrahedral Geometry
Tetrahedral geometry is a molecular shape that arises when a central atom is surrounded by four other atoms or groups of atoms, positioned at the corners of a tetrahedron. In this arrangement, the bond angles are approximately 109.5 degrees, minimizing electron pair repulsion according to VSEPR theory. This geometry is commonly observed in molecules like methane (CH4) and is crucial for understanding the spatial arrangement of ligands around a central atom.
Recommended video:
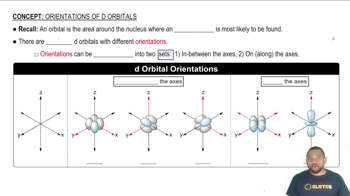
d Orbitals
d orbitals are a set of five atomic orbitals that can hold a maximum of ten electrons. They are important in transition metals and play a significant role in bonding and the formation of complex ions. In tetrahedral coordination, the relevant d orbitals (specifically dxy, dyz, and dxz) are oriented such that their lobes point between the ligands, facilitating effective overlap and bonding interactions.
Recommended video:
Ligand Field Theory
Ligand Field Theory (LFT) is an extension of crystal field theory that describes the electronic structure of transition metal complexes. It considers the effects of ligands on the energy levels of d orbitals, leading to splitting patterns that influence the geometry and reactivity of the complex. In tetrahedral complexes, the d orbitals split into two sets, with the lower-energy orbitals being those that point between the ligands, which is essential for understanding the bonding in such geometries.
Recommended video:
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.