Here are the essential concepts you must grasp in order to answer the question correctly.
Octahedral Geometry
Octahedral geometry is a molecular shape where a central atom is surrounded by six ligands positioned at the vertices of an octahedron. This arrangement allows for optimal spatial distribution of the ligands, minimizing repulsion between them. In this geometry, the bond angles between the ligands are 90 degrees, which is crucial for understanding the spatial orientation of d orbitals.
Recommended video:
d Orbitals
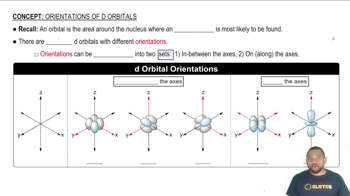
d orbitals are a set of five orbitals in the electron configuration of transition metals, designated as dxy, dyz, dzx, dx2-y2, and dz2. These orbitals have distinct shapes and orientations, influencing how transition metals interact with ligands. In octahedral complexes, the d orbitals split into two energy levels due to ligand field theory, affecting the electronic properties and reactivity of the metal center.
Recommended video:
Ligand Field Theory
Ligand field theory explains the interaction between transition metal ions and surrounding ligands, focusing on how the presence of ligands affects the energy levels of the metal's d orbitals. In octahedral complexes, the ligands cause the d orbitals to split into two groups: the lower-energy t2g orbitals and the higher-energy eg orbitals. This splitting is essential for predicting the electronic configuration, color, and magnetic properties of the complex.
Recommended video:
Strong-Field Ligands result in a large Δ and Weak-Field Ligands result in a small Δ.