Here are the essential concepts you must grasp in order to answer the question correctly.
Diamagnetism
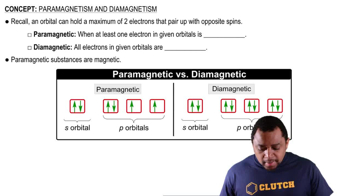
Diamagnetic substances are materials that are not attracted to a magnetic field. They have all their electrons paired, which results in no net magnetic moment. When exposed to a magnetic field, they develop a weak, negative magnetic susceptibility, causing them to be repelled by the field.
Recommended video:
Paramagnetism and Diamagnetism
Paramagnetism
Paramagnetic substances contain unpaired electrons, which give them a net magnetic moment. These materials are attracted to magnetic fields due to the alignment of their unpaired electrons with the external field. This attraction is generally weak and disappears when the external magnetic field is removed.
Recommended video:
Paramagnetism and Diamagnetism
Magnetic Susceptibility
Magnetic susceptibility is a measure of how much a material will become magnetized in an external magnetic field. It quantifies the degree of magnetization of a substance in response to the applied field. Positive susceptibility indicates paramagnetic behavior, while negative susceptibility indicates diamagnetic behavior.
Recommended video:
 Verified step by step guidance
Verified step by step guidance