Here are the essential concepts you must grasp in order to answer the question correctly.
Beta Particle
A beta particle is a high-energy, high-speed electron or positron emitted during the radioactive decay of an atomic nucleus. It is a type of radiation that occurs when a neutron in the nucleus transforms into a proton, emitting an electron (beta-minus) or when a proton transforms into a neutron, emitting a positron (beta-plus).
Recommended video:
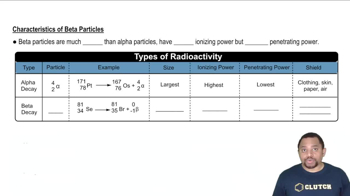
Characteristics of Beta Particles
Symbol for Beta Particle
The symbol for a beta particle is typically represented as 'β' for beta-minus (electron) and 'β+' for beta-plus (positron). These symbols are used in nuclear equations to denote the emission of beta radiation during decay processes.
Recommended video:
Characteristics of Beta Particles
Radioactive Decay
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This can occur in various forms, including alpha, beta, and gamma decay, and is a fundamental concept in nuclear chemistry and physics, influencing the stability and transformation of elements.
Recommended video:
Rate of Radioactive Decay