Textbook Question
Assume that you encounter the following sentences in your reading. What is the chemical formula for each substance mentioned? (c) Hydrogen cyanide is a very poisonous gas.
481
views
 Verified step by step guidance
Verified step by step guidance
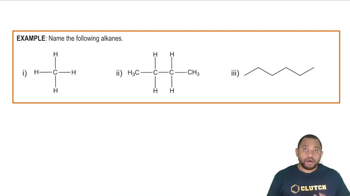

Assume that you encounter the following sentences in your reading. What is the chemical formula for each substance mentioned? (c) Hydrogen cyanide is a very poisonous gas.
(a) What is a hydrocarbon?
(b) Pentane is the alkane with a chain of five carbon atoms. Determine its empirical formula.
(a) What is meant by the term isomer?
(b) Among the four alkanes, ethane, propane, butane, and pentane, which is capable of existing in isomeric forms?
(a) What is a functional group?