Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerism
Isomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations. This can lead to variations in physical and chemical properties. In organic chemistry, isomers can be classified into structural isomers, which differ in the connectivity of atoms, and stereoisomers, which differ in the spatial arrangement of atoms.
Recommended video:
Isomerism in Coordination Complexes Example
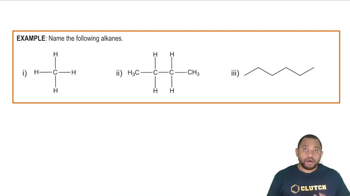
Alkanes
Alkanes are a class of hydrocarbons characterized by single bonds between carbon atoms and a general formula of CnH2n+2. They are saturated compounds, meaning they contain the maximum number of hydrogen atoms per carbon atom. The simplest alkanes include methane, ethane, propane, butane, and pentane, with increasing carbon chain lengths leading to more complex structures.
Recommended video:
Structural Isomers of Alkanes
Structural isomers of alkanes occur when alkanes with the same molecular formula can be arranged in different ways. For example, butane (C4H10) can exist as n-butane and isobutane, while pentane (C5H12) can have three structural isomers: n-pentane, isopentane, and neopentane. The ability to form isomers increases with the number of carbon atoms in the alkane, making it essential to identify which alkanes can exhibit this property.
Recommended video:
 Verified step by step guidance
Verified step by step guidance