(b) Calculate the percent ionization of 0.0075 M butanoic acid in a solution containing 0.085 M sodium butanoate.
Which of the following solutions is a buffer? (a) 0.10 M CH3COOH and 0.10 M CH3COONa, (b) 0.10 M CH3COOH, (c) 0.10 M HCl and 0.10 M NaCl, (d) both a and c, (e) all of a, b, and c.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Buffer Solutions

Weak Acids and Conjugate Bases

Strong Acids vs. Weak Acids
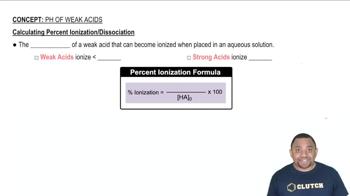
(a) Calculate the percent ionization of 0.125 M lactic acid (Ka = 1.4 × 10-4).
(b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.
Which of the following solutions is a buffer? (a) A solution made by mixing 100 mL of 0.100 M CH3COOH and 50 mL of 0.100 M NaOH, (b) a solution made by mixing 100 mL of 0.100 M CH3COOH and 500 mL of 0.100 M NaOH, (c) A solution made by mixing 100 mL of 0.100 M CH3COOH and 50 mL of 0.100 M HCl, (d) A solution made by mixing 100 mL of 0.100 M CH3COOK and 50 mL of 0.100 M KCl.
(a) Calculate the pH of a buffer that is 0.12 M in lactic acid and 0.11 M in sodium lactate.
(b) Calculate the pH of a buffer formed by mixing 85 mL of 0.13 M lactic acid with 95 mL of 0.15 M sodium lactate.
