Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (Kf)
The equilibrium constant, Kf, is a numerical value that expresses the ratio of the concentrations of products to reactants at equilibrium for a specific reaction. In this context, Kf is used to determine the extent to which a complex ion forms from its constituents in solution. Understanding Kf is essential for calculating the concentrations of ions in equilibrium, as it provides the necessary relationship between the species involved.
Recommended video:
Complex Ion Formation
Complex ion formation occurs when metal ions bond with ligands, resulting in a charged species. In this case, Ni2+ ions interact with NH3 ligands to form the complex ion Ni(NH3)6^2+. Recognizing how ligands coordinate with metal ions is crucial for predicting the concentrations of both free metal ions and complex ions at equilibrium, as it directly influences the calculations involving Kf.
Recommended video:
Complex Ions and Formation Constant
Stoichiometry and Concentration Calculations
Stoichiometry involves the quantitative relationships between reactants and products in a chemical reaction. To find the concentrations of Ni2+ and Ni(NH3)6^2+ at equilibrium, one must first convert the mass of NiCl2 into moles and then use the initial concentrations and the stoichiometric coefficients from the balanced equation. This process is vital for determining how much of each species is present at equilibrium after the reaction has occurred.
Recommended video:
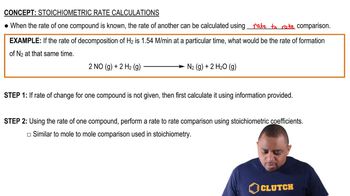
Stoichiometric Rate Calculations
 Verified step by step guidance
Verified step by step guidance