Using data from Appendix D, calculate 3OH-4 and pH for each of the following solutions: (b) 0.035 M Na2S
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (c) A solution of pyridinium bromide has a pH of 2.95. What is the concentration of the pyridinium cation at equilibrium, in units of molarity?

Verified Solution
Key Concepts
Strong Electrolytes

pH and Hydrogen Ion Concentration

Equilibrium Concentration
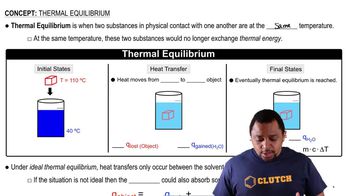
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (a) Write out the reaction that leads to this acidic pH.
Pyridinium bromide 1C5H5NHBr2 is a strong electrolyte that dissociates completely into C5H5NH+ and Br-. An aqueous solution of pyridinium bromide has a pH of 2.95. (b) Using Appendix D, calculate the Ka for pyridinium bromide.
Predict whether aqueous solutions of the following compounds are acidic, basic, or neutral: (c) Na2CO3
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (a) AlCl3
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral: (b) NaBr
