Here are the essential concepts you must grasp in order to answer the question correctly.
Dissociation of Sodium Sulfide (Na2S)
Sodium sulfide (Na2S) dissociates in water to produce sodium ions (Na+) and sulfide ions (S2-). The sulfide ion can further react with water to form hydroxide ions (OH-) and hydrogen sulfide (H2S), leading to an increase in the concentration of OH- in the solution. Understanding this dissociation is crucial for calculating the concentration of hydroxide ions in the solution.
Recommended video:
Percent Dissociation Example
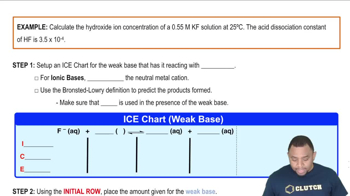
Hydroxide Ion Concentration (OH-)
The concentration of hydroxide ions (OH-) is a key factor in determining the basicity of a solution. In this case, the dissociation of Na2S produces OH- ions, which can be calculated based on the stoichiometry of the reaction. The concentration of OH- is essential for calculating the pH of the solution, as it directly influences the acidity or basicity.
Recommended video:
Hydroxide Ion Concentration Example
pH Calculation
pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm of the hydrogen ion concentration (H+). For basic solutions, pH can be calculated using the relationship between pH and pOH, where pH + pOH = 14. By first determining the concentration of OH- from the dissociation of Na2S, one can find pOH and subsequently calculate the pH of the solution.
Recommended video:
 Verified step by step guidance
Verified step by step guidance