Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) BrO- or BrO2-
Indicate whether each of the following statements is true or false. For each statement that is false, correct the statement to make it true. (a) Acid strength in a series of H¬A molecules increases with increasing size of A. (b) For acids of the same general structure but differing electronegativities of the central atoms, acid strength decreases with increasing electronegativity of the central atom. (c) The strongest acid known is HF because fluorine is the most electronegative element.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
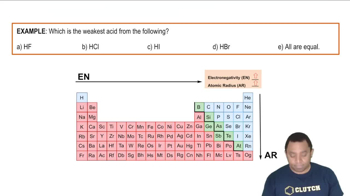
Acid Strength and Atomic Size

Electronegativity and Acid Strength

Strongest Acids and HF
Based on their compositions and structures and on conjugate acid–base relationships, select the stronger base in each of the following pairs: (b) PO43- or AsO43-
Indicate whether each of the following statements is true or false. For each statement that is false, correct the statement to make it true. (a) In general, the acidity of binary acids increases from left to right in a given row of the periodic table. (b) In a series of acids that have the same central atom, acid strength increases with the number of hydrogen atoms bonded to the central atom. (c) Hydrotelluric acid 1H2Te2 is a stronger acid than H2S because Te is more electronegative than S.
Identify the Lewis acid and Lewis base among the reactants in each of the following reactions: (d) HIO1lq2 + NH2-1lq2 Δ NH31lq2 + IO-1lq2(lq denotes liquid ammonia as solvent)
