Here are the essential concepts you must grasp in order to answer the question correctly.
Strong Bases
Strong bases are substances that completely dissociate in water to produce hydroxide ions (OH-). Common examples include alkali metal hydroxides and alkaline earth metal hydroxides, such as calcium hydroxide (Ca(OH)2). Understanding the dissociation of strong bases is crucial for calculating hydroxide ion concentration in solution.
Recommended video:
Strong Acid-Strong Base Titration
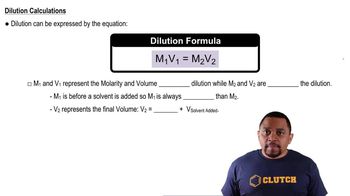
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The dilution equation, C1V1 = C2V2, relates the initial concentration and volume to the final concentration and volume. This concept is essential for determining the new concentration of hydroxide ions after the solution is diluted to a specific volume.
Recommended video:
pH and pOH Calculations
pH and pOH are measures of the acidity and basicity of a solution, respectively. The pH scale ranges from 0 to 14, with lower values indicating higher acidity and higher values indicating higher basicity. The relationship between pH and pOH is given by the equation pH + pOH = 14. For strong bases, calculating pOH from the hydroxide ion concentration allows for the determination of pH.
Recommended video:
 Verified step by step guidance
Verified step by step guidance