Consider the hypothetical reaction A(𝑔) ⇌ 2 B(𝑔). A flask is charged with 0.75 atm of pure A, after which it is allowed to reach equilibrium at 0°C. At equilibrium, the partial pressure of A is 0.36 atm. (c) What could we do to maximize the yield of B?
For the equilibrium PH3BCl3(𝑠) ⇌ PH3(𝑔) + BCl3(𝑔) 𝐾𝑝 = 0.052 at 60 °C. (b) After 3.00 g of solid PH3BCl3 is added to a closed 1.500-L vessel at 60 °C, the vessel is charged with 0.0500 g of BCl3(𝑔). What is the equilibrium concentration of PH3?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Chemical Equilibrium

Equilibrium Constant (Kp)

Stoichiometry and Concentration Calculations
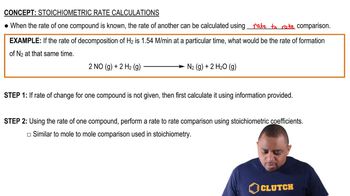
As shown in Table 15.2, the equilibrium constant for the reaction N2(𝑔) + 3 H2(𝑔) ⇌ 2 NH3(𝑔) is 𝐾𝑝 = 4.34×10−3 at 300°C. Pure NH3 is placed in a 1.00-L flask and allowed to reach equilibrium at this temperature. There are 1.05 g NH3 in the equilibrium mixture. (b) What was the initial mass of ammonia placed in the vessel?
A 0.831-g sample of SO3 is placed in a 1.00-L container and heated to 1100 K. The SO3 decomposes to SO2 and O2: 2SO3(𝑔) ⇌ 2 SO2(𝑔) + O2(𝑔) At equilibrium, the total pressure in the container is 1.300 atm. Find the values of 𝐾𝑝 and 𝐾𝑐 for this reaction at 1100 K.
Nitric oxide (NO) reacts readily with chlorine gas as follows: 2 NO(𝑔) + Cl2(𝑔) ⇌ 2 NOCl(𝑔) At 700 K, the equilibrium constant Kp for this reaction is 0.26. Predict the behavior of each of the following mixtures at this temperature and indicate whether or not the mixtures are at equilibrium. If not, state whether the mixture will need to produce more products or reactants to reach equilibrium. (b) PNO = 0.12 atm, PCl2 = 0.10 atm, PNOCl = 0.050 atm
