Enzymes are often described as following the two-step mechanism: E + S Δ ES 1fast2 ES ¡ E + P 1slow2 where E = enzyme, S = substrate, ES = enzyme9substrate complex, and P = product. (b) Molecules that can bind to the active site of an enzyme but are not converted into product are called enzyme inhibitors. Write an additional elementary step to add into the preceding mechanism to account for the reaction of E with I, an inhibitor.
The reaction between ethyl iodide and hydroxide ion in ethanol 1C2H5OH2 solution, C2H5I1alc2 + OH- 1alc2 ¡ C2H5OH1l2 + I - 1alc2, has an activation energy of 86.8 kJ>mol and a frequency factor of 2.10 * 1011 M-1 s-1. (d) Assuming the frequency factor and activation energy do not change as a function of temperature, calculate the rate constant for the reaction at 50 C.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
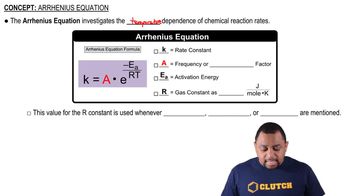
Key Concepts
Arrhenius Equation
Activation Energy

Temperature Conversion

The reaction between ethyl iodide and hydroxide ion in ethanol 1C2H5OH2 solution, C2H5I1alc2 + OH- 1alc2 ¡ C2H5OH1l2 + I - 1alc2, has an activation energy of 86.8 kJ>mol and a frequency factor of 2.10 * 1011 M-1 s-1. (c) Which reagent in the reaction is limiting, assuming the reaction proceeds to completion?
The gas-phase reaction of NO with F2 to form NOF and F has an activation energy of Ea = 6.3 kJ>mol. and a frequency factor of A = 6.0 * 108 M-1 s-1. The reaction is believed to be bimolecular: NO1g2 + F21g2 ¡ NOF1g2 + F1g2 (e) Suggest a reason for the low activation energy for the reaction.
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (a) Calculate the overall standard enthalpy change for the reaction process.
The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (c) Draw a plausible Lewis structure for the intermediate HOOBr. To what familiar compound of hydrogen and oxygen does it appear similar?
