KBr is relatively soluble in water, yet its enthalpy of solution is + 19.8 kJ/mol. Which of the following statements provides the best explanation for this behavior? (a) Potassium salts are always soluble in water. (b) The entropy of mixing must be unfavorable. (c) The enthalpy of mixing must be small compared to the enthalpies for breaking up water–water interactions and K–Br ionic interactions. (d) KBr has a high molar mass compared to other salts like NaCl.
Ch.13 - Properties of Solutions
Chapter 13, Problem 25c
By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution:(c) K2Cr2O7
 Verified step by step guidance
Verified step by step guidance1
Identify the solubility of K_2Cr_2O_7 in water at 40 °C from Figure 13.15. This will tell you how many grams of K_2Cr_2O_7 can dissolve in 100 g of water at this temperature.
Compare the given amount of K_2Cr_2O_7 (40.0 g) to the solubility value obtained from the figure.
If the solubility value is greater than or equal to 40.0 g, the solution will be unsaturated or just saturated, meaning all the K_2Cr_2O_7 can dissolve.
If the solubility value is less than 40.0 g, the solution will be saturated, and some of the K_2Cr_2O_7 will remain undissolved.
Conclude whether the addition of 40.0 g of K_2Cr_2O_7 to 100 g of water at 40 °C results in a saturated solution based on the comparison.

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Solubility
Solubility is the maximum amount of a solute that can dissolve in a given quantity of solvent at a specific temperature. It is typically expressed in grams of solute per 100 grams of solvent. Understanding solubility is crucial for determining whether a solution can become saturated, meaning no more solute can dissolve in the solvent at that temperature.
Recommended video:
Guided course

Solubility Rules
Saturated Solution
A saturated solution is one in which the maximum amount of solute has been dissolved in the solvent at a given temperature, resulting in an equilibrium between dissolved and undissolved solute. When additional solute is added to a saturated solution, it will not dissolve, indicating that the solubility limit has been reached.
Recommended video:
Guided course

Types of Aqueous Solutions
Ionic Compounds and Their Solubility
Ionic compounds, such as K<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub>, dissociate into their constituent ions when dissolved in water. The solubility of these compounds varies widely depending on the specific ions involved and the temperature. Knowledge of the solubility rules helps predict whether a particular ionic solid will dissolve in water and to what extent.
Recommended video:
Guided course
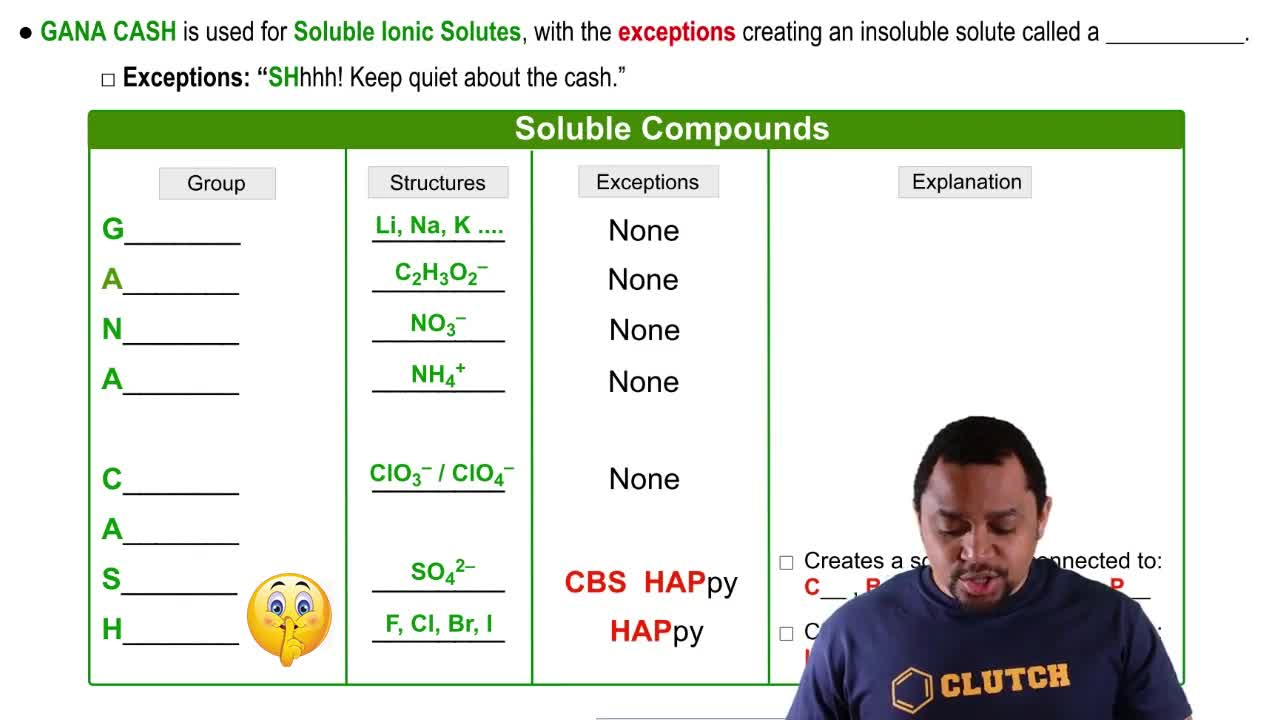
Soluble Ionic Solutes
Related Practice
Textbook Question
2713
views
1
rank
Open Question
The solubility of Cr1NO323 # 9 H2O in water is 208 g per 100 g of water at 15 °C. A solution of Cr1NO323 # 9 H2O in water at 35 °C is formed by dissolving 324 g in 100 g of water. When this solution is slowly cooled to 15 °C, no precipitate forms. (b) You take a metal spatula and scratch the side of the glass vessel that contains this cooled solution, and crystals start to appear. What has just happened?
Open Question
The solubility of MnSO4 · H2O in water at 20 °C is 70 g per 100 mL of water. (b) Given a solution of MnSO4 · H2O of unknown concentration, what experiment could you perform to determine whether the new solution is saturated, supersaturated, or unsaturated?
Textbook Question
By referring to Figure 13.15, determine whether the addition of 40.0 g of each of the following ionic solids to 100 g of water at 40 °C will lead to a saturated solution: (d) Pb(NO3)2.
875
views
Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (b) Pb(NO3)2,
436
views
Textbook Question
By referring to Figure 13.15, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 °C: (c) Ce2(SO4)3.
967
views
2
rank
