At ordinary body temperature (37 °C), the solubility of N2 in water at ordinary atmospheric pressure (1.0 atm) is 0.015 g/L. Air is approximately 78 mol % N2. (b) At a depth of 100 ft in water, the external pressure is 4.0 atm. What is the solubility of N2 from air in blood at this pressure?
A series of anions is shown below:

The anion on the far right is called 'BARF' by chemists, as its common abbreviation sounds similar to this word. (b) What is the electron-domain geometry around the central B in BARF?

Verified Solution
Key Concepts
Electron-Domain Geometry

VSEPR Theory
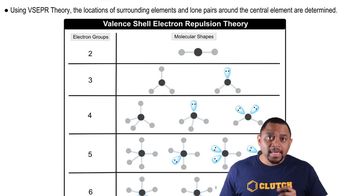
Central Atom Hybridization

A series of anions is shown below:
The anion on the far right is called 'BARF' by chemists, as its common abbreviation sounds similar to this word. (a) What is the central atom and the number of electronpair domains around the central atom in each of these anions?
A series of anions is shown below: The anion on the far right is called 'BARF' by chemists, as its common abbreviation sounds similar to this word. (c) Which, if any, of these anions has an expanded octet around its central atom?
Compounds like sodium stearate, called 'surfactants' in general, can form structures known as micelles in water, once the solution concentration reaches the value known as the critical micelle concentration (cmc). Micelles contain dozens to hundreds of molecules. The cmc depends on the substance, the solvent, and the temperature. (a) The turbidity (the amount of light scattering) of solutions increases dramatically at the cmc. Suggest an explanation. .
