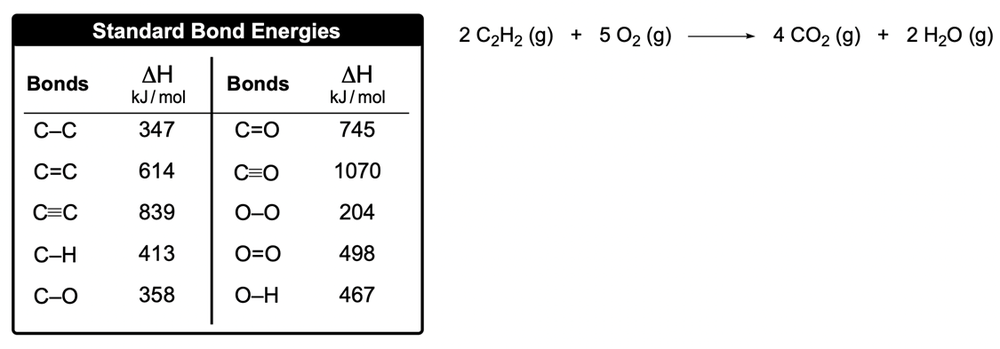
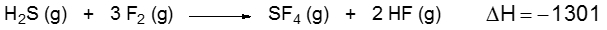Now here I'm going to say that bond energy, also called bond enthalpy and it's represented by ΔHBE, is the amount of energy stored in a bond between atoms in a molecule. Now realize that bond energy values can be calculated and can be used to calculate the enthalpy of reaction, which is ΔH of reaction. Also what's called the heat of reaction at times as well. So just remember it's the enthalpy or heat of reaction.
Now we have endothermic processes versus exothermic processes. In an endothermic process, energy is absorbed to break a bond. And since we're taking in energy, we're gaining energy, it has a positive sign. In an exothermic process, energy is released, lost, and if you're losing energy you have a negative sign. Now if we take a look here, we're accustomed to seeing equations like this.
These two equations we need to remember. We're going to say when given individual bond enthalpies or bond energies, then the enthalpy of reaction formula becomes this: enthalpy of reaction equals reactants minus products. So again, if they're giving us individual bond enthalpies or bond energies, we use this version of the formula.
Now if you've looked at my videos in terms of thermochemistry, you would see that that formula seems kind of familiar. Well, when we're given the enthalpy of formation for a whole compound, then it's products minus reactants. So if you've seen my videos in thermochemistry, you will remember this. If you haven't yet, don't worry about it just yet. Realize that when we're dealing with bond enthalpies, bond energies, it's reactants minus products.