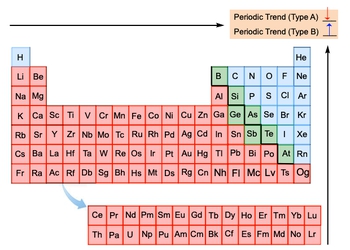
All right. So now let's do a quick review of all the periodic trends. They can be grouped together. Remember, in all of them, we're moving to the top right corner of the periodic table. And we're willing to say here that some of them can be grouped together.
We're going to call first our periodic trends type A or trend A. This is involving metallic character and atomic radius. As we move to the top right corner for them, we're going to say that they decrease. So type A periodic trends decrease as we head towards the top right corner.
And then here the next one which are type B ones. These periodic trends start with either I or E. So that includes ionization energy, electron affinity, electronegativity, and effective nuclear charge. For them, as we head to the top right corner, they increase.
Now we're going to say since ionic radius depends on the number of electrons, it doesn't belong to either group. So here this is a great way to help you put all your periodic trends on one chart and quickly be able to tell what's happening with each one. So just remember your type A and then your type B periodic trends.