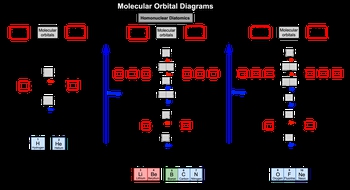
Now recall that a whole nuclear diatomic molecule is composed of two identical elements bonded together. Good example of this is N2 or F2. Now as a result of increase in electronegativity and a decrease in atomic size, the order of Σ2P and Π2P will be reversed for oxygen to neon.
So if we take a look here at molecular orbital diagrams in this first one this correlates to only H2 to HE2. So hydrogen and diatomic helium which is not a normal structure. And remember we're looking at the valence electrons for these elements. In hydrogen and helium their valence electrons are found into 1S orbitals or in. One of the periodic table. So here we'd have for instance, this could be the atomic orbital of hydrogen or helium and the other hydrogen or helium. We would fill in the atomic orbitals and then distribute those electrons into our molecular orbital sphere. Remember Σ1S represents our bonding molecular orbital and Σ*1S represents our antibody molecular orbital.
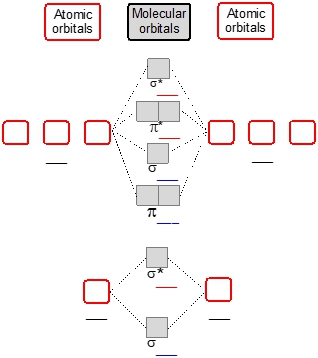
Now if we move to the next one, this correlates to diatomic lithium all the way to diatomic nitrogen. Here it gets more complex because now we're dealing with period two elements. So we'd start off with 2S and we move our way up to 2P. Now here with the 2S orbitals atomic orbitals, we fill in first our bonding molecular orbital which is Σ2S and then start filling in our Σ*2S which represents our antibody molecular orbital. When we get to 2P gets more complex as the atomic orbitals continue to pull their electrons together to create new molecular orbitals. Here the order forward would be Π2P Σ2P. Then it'll go Π*2P which is an antibody molecular orbital to Σ*2P another antibody molecular orbital. Again, we always fill from lowest molecular orbital up.
Now again, because of an increase in electronegativity and a decrease in atomic size, we're going to have a slight change when it comes to diatomic oxygen to diatomic neon. If we look the change happens here they flip. So now my Π2P moves up and my Σ2P moved down South. Here again, oxygen 2 neon. There's still period two elements. So we're starting out with two S again and we just start pulling together our atomic orbital electrons and dumping them into our molecular orbitals and start filling it up as we move up.
So just keep in mind these are the different types of molecular orbitals that can exist based on what type of. Element. You're dealing with 1-2 or three. All right. So keep this in mind as we start doing more and more questions dealing with homonuclear diatomic molecules.