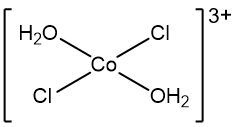
Now when it comes to a complex ion, what is it? Well, a complex ion is an overall charged adduct. So remember, it's charged because it has the name ion in it. It's positive or negative that specifically has a transition metal cation at its center, covently bonded to a ligand or ligand or multiple versions of it. Now this is the definition we're going to use for our Gen. Chem course.
Now of course there are adducts that could have main Grou elements instead of a transition metal at the center, but those we tend not to see, we're going to say the complex ion. A dead giveaway to recognize it is that it's always written in brackets. So we start looking at more complex formulas and part of those formulas will contain complex ions. You can always spot them by the portion that's in brackets.
Now here an example of a complex ion is shown as. So here we have our metal cation in the form of copper 3 ion and our ligands here are 4 ammonias. Those four ammonias will attach themselves to the copper 3 ion, so we're going to draw them here. And remember the overall charge of our adduct comes from the combined charges of our metal cation and all the ligands together.
So the copper metal has a three plus charge, ammonia four of them, they're all neutral. So they have no charge. So the overall charge here of our add a product or our complex ion now is 3 plus. So remember we are combining these structures together, we're adding them together. So that's why it's called an adduct, but another name for this adduct is our complex ion. So the complex ion comes from combining together the metal cation with some set number of ligands, so keep that in mind.