As discussed in the A Closer Look box on 'Measurement and the Uncertainty Principle,' the essence of the uncertainty principle is that we can't make a measurement without disturbing the system that we are measuring. (a) Why can't we measure the position of a subatomic particle without disturbing it?
Ch.6 - Electronic Structure of Atoms
Chapter 6, Problem 100c
The Chemistry and Life box in Section 6.7 described the techniques called NMR and MRI. (c) When the 450-MHz photon is absorbed, does it change the spin of the electron or the proton on a hydrogen atom?
 Verified step by step guidance
Verified step by step guidance1
Understand the context: NMR (Nuclear Magnetic Resonance) and MRI (Magnetic Resonance Imaging) are techniques that involve the interaction of magnetic fields with atomic nuclei, particularly focusing on hydrogen atoms.
Identify the key component: In NMR, the focus is on the nucleus of the hydrogen atom, which is a proton. The technique involves the absorption of radiofrequency photons by the nucleus.
Consider the frequency: The 450-MHz photon mentioned is a radiofrequency photon, which is typically used in NMR to interact with the nuclear spins, not electron spins.
Determine the effect: When a 450-MHz photon is absorbed in NMR, it causes a transition in the spin state of the proton (nucleus) in the hydrogen atom, not the electron.
Conclude the understanding: Therefore, the absorption of a 450-MHz photon in NMR changes the spin of the proton in the hydrogen atom.
Recommended similar problem, with video answer:

Verified Solution
This video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR)
Nuclear Magnetic Resonance (NMR) is a spectroscopic technique that exploits the magnetic properties of certain atomic nuclei. When placed in a magnetic field, nuclei such as protons can absorb specific frequencies of electromagnetic radiation, leading to transitions between different energy states. This process is fundamental in determining the structure of organic compounds and is the basis for techniques like MRI in medical imaging.
Recommended video:
Guided course

Resonance Structures
Spin of Nuclei
Spin is a fundamental property of particles, including protons and electrons, which can be thought of as a form of intrinsic angular momentum. In the context of NMR, the spin of the proton in a hydrogen atom is particularly important, as it determines how the nucleus interacts with magnetic fields. When a photon is absorbed, it can cause a change in the orientation of the spin state of the proton, but not the electron.
Recommended video:
Guided course
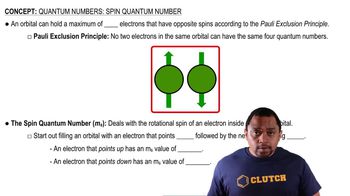
Spin Quantum Number
Photon Absorption
Photon absorption occurs when an atom or molecule takes in a photon, resulting in an increase in energy. In NMR, when a photon of a specific frequency (like 450 MHz) is absorbed by a hydrogen atom, it can promote the proton from a lower energy spin state to a higher energy state. This process does not affect the electron's spin, as the energy levels and transitions involved in NMR primarily pertain to the nuclear spins.
Recommended video:
Guided course

Moles and Photon Energy
Related Practice
Textbook Question
478
views
Textbook Question
Consider the discussion of radial probability functions in
'A Closer Look' in Section 6.6. (a) What is the difference
between the probability density as a function of r and the
radial probability function as a function of r ?
389
views
Textbook Question
The Chemistry and Life box in Section 6.7 described the techniques called NMR and MRI. (a) Instruments for obtaining MRI data are typically labeled with a frequency, such as 600 MHz. In what region of the electromagnetic spectrum does a photon with this frequency belong?
Open Question
Suppose that the spin quantum number, ms, could have three allowed values instead of two. How would this affect the number of elements in the first four rows of the periodic table?
Textbook Question
Using the periodic table as a guide, write the condensed electron configuration and determine the number of unpaired electrons for the ground state of (a) Cl (b) Al (c) Zr (d) As (e) Sb (f) W.
573
views
Textbook Question
Scientists have speculated that element 126 might have a moderate stability, allowing it to be synthesized and characterized. Predict what the condensed electron configuration of this element might be.
1418
views
