Provide a brief explanation for each of the following.
K+ is larger than Na+.
 Verified step by step guidance
Verified step by step guidance


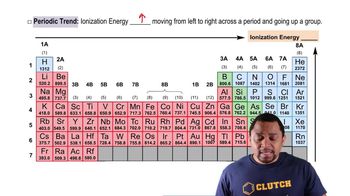
Provide a brief explanation for each of the following.
K+ is larger than Na+.
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 201 pm (Li–F), 282 pm (Na–Cl), 330 pm (K–Br), and 367 pm (Rb–I), respectively. (b) Calculate the difference between the experimentally measured ion–ion distances and the ones predicted from Figure 7.8.
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 201 pm (Li–F), 282 pm (Na–Cl), 330 pm (K–Br), and 367 pm (Rb–I), respectively. (c) What estimates of the cation– anion distance would you obtain for these four compounds using neutral atom bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
Which element has the highest second ionization energy: Li, K, or Be?