Here are the essential concepts you must grasp in order to answer the question correctly.
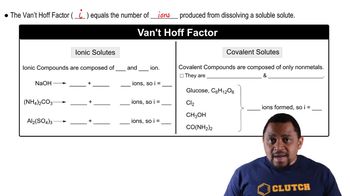
van't Hoff Factor (i)
The van't Hoff factor (i) is a measure of the number of particles a solute dissociates into when dissolved in a solvent. For example, sodium chloride (NaCl) dissociates into two ions, Na+ and Cl-, giving it a van't Hoff factor of 2. This factor is crucial for calculating colligative properties, such as boiling point elevation and freezing point depression, as it directly affects the extent of these changes in solution.
Recommended video:
Colligative Properties
Colligative properties are properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. Key examples include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. Understanding these properties is essential for solving problems related to the effects of solutes on solvent behavior, as seen in the question regarding melting and boiling points.
Recommended video:
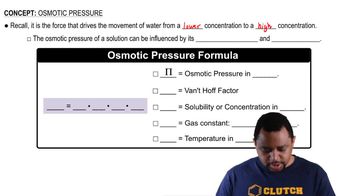
Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane, and it is directly proportional to the concentration of solute particles in the solution. The formula for osmotic pressure (π) is π = iCRT, where i is the van't Hoff factor, C is the molarity of the solution, R is the ideal gas constant, and T is the temperature in Kelvin. This concept is vital for calculating the mass of solute needed to achieve a specific osmotic pressure.
Recommended video: