Here are the essential concepts you must grasp in order to answer the question correctly.
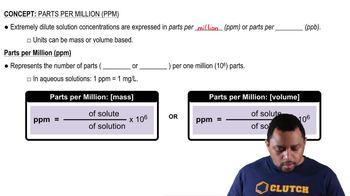
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement used to express very dilute concentrations of substances. It indicates how many parts of a substance are present in one million parts of a solution or mixture. For example, 1 ppm means 1 part of solute in 1,000,000 parts of solution, making it a useful measure for trace elements in ores or environmental samples.
Recommended video:
Conversion of Mass to ppm
To convert a mass concentration to ppm, the mass of the substance is divided by the total mass of the solution or mixture, then multiplied by 1,000,000. In this case, since the ore contains silver, the total mass is considered in terms of grams per ton, where 1 ton equals 1,000,000 grams, facilitating the conversion to ppm.
Recommended video:
Energy to Mass Conversion
Understanding Ore Composition
Ore composition refers to the specific elements and their concentrations found within a mineral deposit. In this context, knowing the amount of silver in the ore allows for calculations of its economic viability and extraction potential. Understanding the composition is crucial for mining and metallurgy, as it influences processing methods and costs.
Recommended video: