Textbook Question
Indicate whether each statement is true or false: (c) As you cool a saturated solution from high temperature to low temperature, solids start to crystallize out of solution if you achieve a supersaturated solution.
 Verified step by step guidance
Verified step by step guidance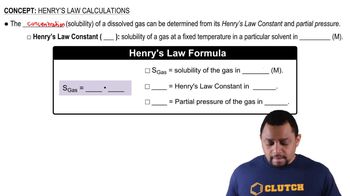


Indicate whether each statement is true or false: (c) As you cool a saturated solution from high temperature to low temperature, solids start to crystallize out of solution if you achieve a supersaturated solution.
Indicate whether each statement is true or false: (d) If you take a saturated solution and raise its temperature, you can (usually) add more solute and make the solution even more concentrated.
(a) Calculate the mass percentage of Na2SO4 in a solution containing 10.6 g of Na2SO4 in 483 g of water.
(b) An ore contains 2.86 g of silver per ton of ore. What is the concentration of silver in ppm?