Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Acids and Bases
Lewis acids are defined as electron pair acceptors, while Lewis bases are electron pair donors. This concept expands the traditional Brønsted-Lowry definitions of acids and bases, allowing for a broader range of chemical interactions. Understanding this distinction is crucial for identifying which species in a reaction can act as an acid or a base.
Recommended video:
Electron Deficiency
Electron deficiency refers to the lack of a complete octet in an atom, making it more reactive and capable of accepting electron pairs. In the context of Lewis acids, species like BF3 are electron-deficient due to the presence of an empty p-orbital, which enhances their ability to act as Lewis acids compared to those with a complete octet, like BH3.
Recommended video:
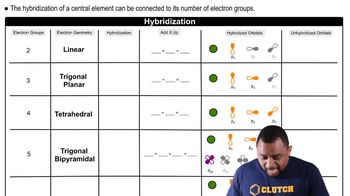
Molecular Geometry and Hybridization
The molecular geometry and hybridization of a compound influence its reactivity and acid-base behavior. BF3 has a trigonal planar geometry with sp2 hybridization, which allows for effective orbital overlap when accepting electron pairs. In contrast, BH3, with its similar geometry, is less electron-deficient, making BF3 the stronger Lewis acid in this comparison.
Recommended video:
Hybridization and Electron Geometry
 Verified step by step guidance
Verified step by step guidance