Most fish need at least 4 ppm dissolved O2 in water for survival. (a) What is this concentration in mol/L?
Ch.13 - Properties of Solutions
Chapter 13, Problem 94b
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (b) A sample consisting of various gases contains 3.5 × 10-6 mole fraction of radon. This gas at a total pressure of 32 atm is shaken with water at 30 °C. Calculate the molar concentration of radon in the water.
 Verified step by step guidance
Verified step by step guidance1
First, understand that the problem involves calculating the molar concentration of radon in water using Henry's Law, which relates the solubility of a gas in a liquid to the partial pressure of the gas above the liquid.
Identify the given values: the mole fraction of radon in the gas mixture is 3.5 × 10<sup>-6</sup>, and the total pressure of the gas mixture is 32 atm.
Calculate the partial pressure of radon using the formula: <math xmlns='http://www.w3.org/1998/Math/MathML'><mrow><msub><mi>P</mi><mi>Rn</mi></msub><mo>=</mo><msub><mi>X</mi><mi>Rn</mi></msub><mo>×</mo><mi>P</mi></mrow></math>, where <math xmlns='http://www.w3.org/1998/Math/MathML'><msub><mi>X</mi><mi>Rn</mi></msub></math> is the mole fraction of radon and <math xmlns='http://www.w3.org/1998/Math/MathML'><mi>P</mi></math> is the total pressure.
Use Henry's Law to find the molar concentration of radon in water: <math xmlns='http://www.w3.org/1998/Math/MathML'><mrow><msub><mi>C</mi><mi>Rn</mi></msub><mo>=</mo><msub><mi>k</mi><mi>H</mi></msub><mo>×</mo><msub><mi>P</mi><mi>Rn</mi></msub></mrow></math>, where <math xmlns='http://www.w3.org/1998/Math/MathML'><msub><mi>k</mi><mi>H</mi></msub></math> is the Henry's Law constant for radon at 30 °C.
Look up the value of the Henry's Law constant for radon at 30 °C in a reliable source, then substitute the values into the equation to find the molar concentration of radon in the water.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Mole Fraction
Mole fraction is a way of expressing the concentration of a component in a mixture. It is defined as the ratio of the number of moles of a specific component to the total number of moles of all components in the mixture. In this case, the mole fraction of radon indicates its proportion relative to other gases present, which is crucial for calculating its concentration in water.
Recommended video:
Guided course

Mole Fraction Formula
Henry's Law
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This principle is essential for determining how much radon will dissolve in water when it is in contact with the gas at a specific pressure, allowing us to calculate its molar concentration in the water.
Recommended video:
Guided course
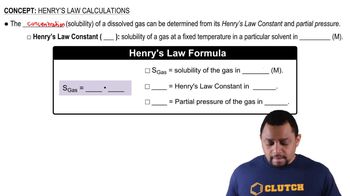
Henry's Law Calculations
Molar Concentration
Molar concentration, or molarity, is defined as the number of moles of solute per liter of solution. It provides a measure of how concentrated a solution is and is critical for understanding the amount of radon that can be expected to dissolve in water under the given conditions. This concept is key to solving the problem by converting the amount of radon from its mole fraction and pressure into a concentration value.
Recommended video:
Guided course

Molarity Concept
Related Practice
Textbook Question
908
views
Textbook Question
Most fish need at least 4 ppm dissolved O2 in water for survival. (b) What partial pressure of O2 above water is needed to obtain 4 ppm O2 in water at 10 °C? (The Henry's law constant for O2 at this temperature is 1.71⨉10-3 mol/L-atm.)
597
views
Textbook Question
The presence of the radioactive gas radon (Rn) in well water presents a possible health hazard in parts of the United States. (a) Assuming that the solubility of radon in water with 1 atm pressure of the gas over the water at 30 °C is 7.27⨉10-3 M, what is the Henry's law constant for radon in water at this temperature?
579
views
1
rank
Open Question
Glucose makes up about 0.10% by mass of human blood. Calculate this concentration in molality. What further information would you need to determine the molarity of the solution?
Open Question
The concentration of gold in seawater has been reported to be between 5 ppt (parts per trillion) and 50 ppt. Assuming that seawater contains 13 ppt of gold, calculate the number of grams of gold contained in 1.0 * 10^3 gal of seawater.
Textbook Question
The maximum allowable concentration of lead in drinking water is 9.0 ppb. (a) Calculate the molarity of lead in a 9.0-ppb solution.
