Calculate the number of kilowatt-hours of electricity required to produce 1.0 * 103 kg (1 metric ton) of aluminum by electrolysis of Al3+ if the applied voltage is 4.50 V and the process is 45% efficient.
Ch.20 - Electrochemistry
Chapter 20, Problem 113b
Aqueous solutions of ammonia 1NH32 and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: 'Never mix ammonia and bleach, as toxic gases may be produced.' One of the toxic gases that can be produced is chloroamine, NH2Cl. (b) What is the oxidation number of chlorine in chloramine?
 Verified step by step guidance
Verified step by step guidance1
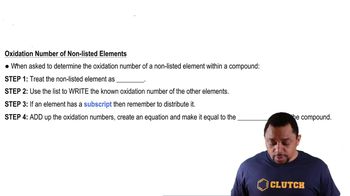
Identify the chemical formula of chloramine, which is NH_2Cl.
Recall that the sum of oxidation numbers in a neutral compound is zero.
Assign oxidation numbers to hydrogen and nitrogen: hydrogen (H) is +1 and nitrogen (N) is typically -3 in compounds.
Set up an equation using the known oxidation numbers: 2(+1) + (-3) + x = 0, where x is the oxidation number of chlorine (Cl).
Solve the equation for x to find the oxidation number of chlorine in chloramine.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation Number
The oxidation number, or oxidation state, is a concept used to indicate the degree of oxidation of an atom in a compound. It represents the hypothetical charge an atom would have if all bonds were ionic. Oxidation numbers are assigned based on a set of rules, including the fact that the oxidation number of an element in its elemental form is zero, and for monoatomic ions, it equals the charge of the ion.
Recommended video:
Guided course

Oxidation Numbers
Chloroamine Structure
Chloroamine, specifically NH2Cl, is a compound that contains nitrogen, hydrogen, and chlorine. In this molecule, the nitrogen atom is bonded to two hydrogen atoms and one chlorine atom. Understanding the structure of chloroamine is essential for determining the oxidation state of chlorine, as it helps visualize how the atoms are arranged and how they interact with each other.
Recommended video:
Guided course

Resonance Structures
Determining Oxidation States
To determine the oxidation state of chlorine in chloroamine, one must consider the known oxidation states of the other atoms in the molecule. Nitrogen typically has an oxidation state of -3 when bonded to hydrogen, and hydrogen has an oxidation state of +1. By applying the rule that the sum of oxidation states in a neutral compound must equal zero, one can calculate the oxidation state of chlorine in NH2Cl.
Recommended video:
Guided course
Determining Oxidation Numbers
Related Practice
Textbook Question
980
views
Textbook Question
The Haber process is the principal industrial route for converting nitrogen into ammonia: N2(g) + 3 H2(g) → 2 NH3(g) (b) Using the thermodynamic data in Appendix C, calculate the equilibrium constant for the process at room temperature.
717
views
Open Question
In a galvanic cell, the cathode is an Ag+ | 1.00 M | Ag(s) half-cell. The anode is a standard hydrogen electrode immersed in a buffer solution containing 0.10 M benzoic acid (C6H5COOH) and 0.050 M sodium benzoate (C6H5COO-Na+). The measured cell voltage is 1.030 V. What is the pKa of benzoic acid?
Textbook Question
Aqueous solutions of ammonia 1NH32 and bleach (active ingredient NaOCl) are sold as cleaning fluids, but bottles of both of them warn: 'Never mix ammonia and bleach, as toxic gases may be produced.' One of the toxic gases that can be produced is chloroamine, NH2Cl. (e) Is N oxidized, reduced, or neither, upon the conversion of ammonia to nitrogen trichloride?
347
views
Open Question
A voltaic cell is based on Ag+ (aq) > Ag (s) and Fe3+ (aq) > Fe2+ (aq) half-cells. Use S° values in Appendix C and the relationship between cell potential and free-energy change to predict whether the standard cell potential increases or decreases when the temperature is raised above 25 °C.
Open Question
Hydrogen gas has the potential for use as a clean fuel in reaction with oxygen. The relevant reaction is 2 H2(g) + O2(g) → 2 H2O(l). Consider two possible ways of utilizing this reaction as an electrical energy source: (i) Hydrogen and oxygen gases are combusted and used to drive a generator, much as coal is currently used in the electric power industry; (ii) hydrogen and oxygen gases are used to generate electricity directly by using fuel cells that operate at 85 °C. Based on the analysis here, would it be more efficient to use the combustion method or the fuel-cell method to generate electrical energy from hydrogen?
