Write equations for the half-reactions that occur at the anode and cathode for the electrolysis of each aqueous solution. a. Ni(NO3)2(aq)
Ch.19 - Electrochemistry
Chapter 19, Problem 98
How can one sketch an electrolysis cell that electroplates nickel onto other metal surfaces, labeling the anode and cathode and indicating the reactions that occur at each?
 Verified step by step guidance
Verified step by step guidance1
Identify the components of the electrolysis cell: anode, cathode, electrolyte solution, and power source.
Label the anode as the positive electrode where oxidation occurs, and the cathode as the negative electrode where reduction occurs.
Indicate that the anode is made of nickel, which will dissolve into the electrolyte solution as Ni^2+ ions.
Label the cathode as the metal surface to be plated with nickel, where Ni^2+ ions from the solution will gain electrons and deposit as solid nickel.
Write the half-reactions: at the anode, \( \text{Ni (s)} \rightarrow \text{Ni}^{2+} (aq) + 2e^- \); at the cathode, \( \text{Ni}^{2+} (aq) + 2e^- \rightarrow \text{Ni (s)} \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In an electrolysis cell, an external power source applies voltage to facilitate the movement of ions in an electrolyte solution, leading to chemical changes at the electrodes. This process is fundamental in electroplating, where metal ions are deposited onto a surface.
Recommended video:
Guided course

The Electrolytic Cell
Anode and Cathode
In an electrolysis cell, the anode is the electrode where oxidation occurs, meaning it loses electrons, while the cathode is where reduction takes place, gaining electrons. For nickel electroplating, the anode typically consists of nickel metal, which dissolves into the solution as nickel ions, while the cathode is the surface being plated, where nickel ions are reduced and deposited.
Recommended video:
Guided course

Electrolytic Cell Components Example
Half-Reactions
Half-reactions are the individual oxidation and reduction reactions that occur at the anode and cathode, respectively. In the context of nickel electroplating, the half-reaction at the anode involves the oxidation of nickel metal to nickel ions, while the cathode reaction involves the reduction of nickel ions back to solid nickel. Understanding these half-reactions is crucial for sketching the electrolysis cell accurately.
Recommended video:
Guided course
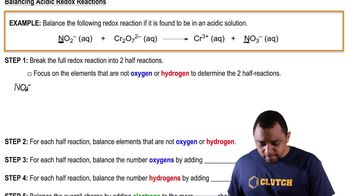
Redox Half Reactions Example
Related Practice
Textbook Question
3028
views
Textbook Question
Write equations for the half-reactions that occur at the anode and cathode for the electrolysis of each aqueous solution. b. KCl(aq) c. CuBr2(aq)
Textbook Question
Make a sketch of an electrolysis cell that electroplates copper onto other metal surfaces. Label the anode and the cathode and indicate the reactions that occur at each.
886
views
Open Question
Copper can be electroplated at the cathode of an electrolysis cell by the half-reaction: Cu2+(aq) + 2 e- → Cu(s). How much time would it take for 325 mg of copper to be plated at a current of 5.6 A?
Textbook Question
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction: Ag+(aq) + e– → Ag(s) What mass of silver would plate onto the cathode if a current of 6.8 A flowed through the cell for 72 min?
2188
views
Textbook Question
A major source of sodium metal is the electrolysis of molten sodium chloride. What magnitude of current produces 1.0 kg of sodium metal in 1 hour?
1055
views
