Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In this process, an electric current is passed through an electrolyte, causing the movement of ions towards the electrodes. At the cathode, reduction occurs, where cations gain electrons and are deposited as solid metal, as seen in the silver plating reaction.
Recommended video:
Faraday's Laws of Electrolysis
Faraday's Laws of Electrolysis quantify the relationship between the amount of substance deposited during electrolysis and the electric charge passed through the cell. The first law states that the mass of a substance deposited is directly proportional to the total electric charge. This relationship is crucial for calculating the mass of silver that can be plated based on the current and time.
Recommended video:
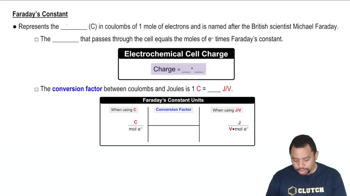
Faraday's Constant in Electrochemistry
Current and Charge
Current, measured in amperes (A), is the flow of electric charge per unit time. To find the total charge (Q) that flows through the electrolysis cell, the formula Q = I × t is used, where I is the current and t is the time in seconds. This total charge is essential for determining how much silver can be deposited at the cathode during the electrolysis process.
Recommended video:
 Verified step by step guidance
Verified step by step guidance