Which evaporates more quickly: 55 mL of water (H2O) in a beaker or 100 mL of acetone [(CH3)2CO] in an identical beaker under identical conditions? Is the vapor pressure of the two substances different? Explain.
Ch.11 - Liquids, Solids & Intermolecular Forces
Chapter 11, Problem 57
The human body obtains 915 kJ of energy from a candy bar. If this energy were used to vaporize water at 100.0 °C, how much water (in liters) could be vaporized? (Assume the density of water is 1.00 g/mL.)
 Verified step by step guidance
Verified step by step guidance1
Identify the energy required to vaporize water, which is the heat of vaporization (\(\Delta H_{vap}\)) for water at 100.0 °C. This value is typically 40.7 kJ/mol.
Convert the energy obtained from the candy bar from kilojoules to joules. Since 1 kJ = 1000 J, multiply 915 kJ by 1000 to get the energy in joules.
Calculate the number of moles of water that can be vaporized using the energy from the candy bar. Use the formula: \(\text{moles of water} = \frac{\text{energy in joules}}{\Delta H_{vap} \times 1000}\), where \(\Delta H_{vap}\) is in kJ/mol.
Convert the moles of water to grams using the molar mass of water (18.02 g/mol). Use the formula: \(\text{mass of water (g)} = \text{moles of water} \times 18.02\, \text{g/mol}\).
Convert the mass of water in grams to volume in liters. Since the density of water is 1.00 g/mL, the volume in mL is equal to the mass in grams. Convert mL to liters by dividing by 1000.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Heat of Vaporization
The heat of vaporization is the amount of energy required to convert a unit mass of a liquid into vapor without a change in temperature. For water, this value is approximately 2260 J/g at 100 °C. Understanding this concept is crucial for calculating how much water can be vaporized using a given amount of energy.
Recommended video:
Guided course

Heat Capacity
Energy Conversion
Energy conversion involves changing energy from one form to another. In this context, the energy obtained from the candy bar (915 kJ) must be converted into joules (1 kJ = 1000 J) to match the units used for the heat of vaporization. This step is essential for accurately determining the amount of water that can be vaporized.
Recommended video:
Guided course

Conversion Factors
Density of Water
Density is defined as mass per unit volume, and for water, it is typically 1.00 g/mL at standard conditions. This property allows for the conversion between mass and volume, which is necessary when calculating how many liters of water can be vaporized based on the mass of water that corresponds to the energy provided.
Recommended video:
Guided course
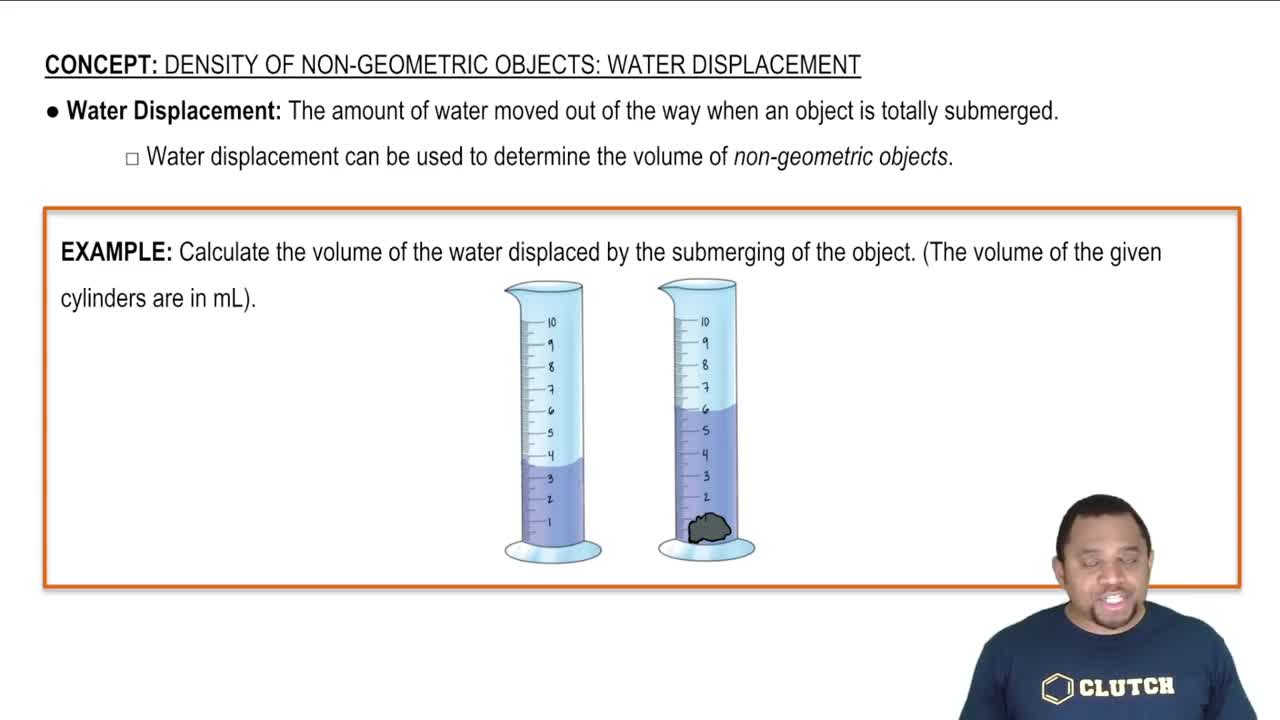
Density via Water Displacement
Related Practice
Textbook Question
951
views
Open Question
Why does spilling room-temperature water over your skin on a hot day cool you down while spilling room-temperature vegetable oil does not?
Open Question
Why is the heat of vaporization of water greater at room temperature than at its boiling point?
Open Question
A 100.0-mL sample of water is heated to its boiling point. How much heat (in kJ) is required to vaporize it? (Assume a density of 1.00 g/mL.)
Textbook Question
Suppose that 0.95 g of water condenses on a 75.0-g block of iron that is initially at 22 °C. If the heat released during condensation goes only to warming the iron block, what is the final temperature (in °C) of the iron block? (Assume a constant enthalpy of vaporization for water of 44.0 kJ/mol.)
4560
views
5
rank
2
comments
Textbook Question
This table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of ammonia.
Temperature (K) Pressure (torr)
200 65.3
210 134.3
220 255.7
230 456.0
235 597.0
2529
views
1
comments
