(a) What is the difference between a CFC and an HFC?
One of the possible consequences of climate change is an increase in the temperature of ocean water. The oceans serve as a 'sink' for CO2 by dissolving large amounts of it.
(a) The figure below shows the solubility of CO2 in water as a function of temperature. Does CO2 behave more or less similarly to other gases in this respect?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
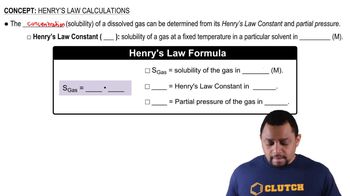
Gas Solubility and Temperature

Henry's Law
Impact of Climate Change on Ocean Chemistry

Natural gas consists primarily of methane, CH4(g). (b) Write a balanced chemical equation for the incomplete combustion of methane to product CO(g) as the only carbon-containg product.
The rate of solar energy striking Earth averages 168 watts per square meter. The rate of energy radiated from Earth's surface averages 390 watts per square meter. Comparing these numbers, one might expect that the planet would cool quickly, yet it does not. Why not?
The solar power striking Earth every day averages 168 watts per square meter. The highest ever recorded electrical power usage in New York City was 13,200 MW. A record established in July of 2013. Considering that present technology for solar energy conversion is about 10% efficient, from how many square meters of land must sunlight be collected in order to provide this peak power? (For compar- ison, the total area of New York City is 830 km2.)
Write balanced chemical equations for each of the following reactions: (b) The nitric oxide molecule undergoes photoionization in the upper atmosphere.
