Which of the following factors would tend to increase membrane fluidity? a. a greater proportion of unsaturated phospholipids b. a greater proportion of saturated phospholipids c. a lower temperature d. a relatively high protein content in the membrane
Ch. 7 - Membrane Structure and Function
Chapter 7, Problem 6
DRAW IT An artificial 'cell' consisting of an aqueous solution enclosed in a selectively permeable membrane is immersed in a beaker containing a different solution, the 'environment,' as shown in the accompanying diagram. The membrane is permeable to water and to the simple sugars glucose and fructose but impermeable to the disaccharide sucrose. a. Draw solid arrows to indicate the net movement of solutes into and/or out of the cell. b. Is the solution outside the cell isotonic, hypotonic, or hypertonic? c. Draw a dashed arrow to show the net osmosis, if any. d. Will the artificial cell become more flaccid, more turgid, or stay the same? e. Eventually, will the two solutions have the same or different solute concentrations?

Verified Solution
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Selective Permeability
Selective permeability refers to the property of biological membranes that allows certain molecules to pass through while blocking others. In this scenario, the membrane permits the passage of water and simple sugars like glucose and fructose, but not larger molecules such as sucrose. This concept is crucial for understanding how substances move in and out of the artificial cell and how it interacts with its environment.
Recommended video:

Natural Selection
Osmosis
Osmosis is the movement of water across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This process is vital for maintaining cell turgor and overall homeostasis. In the context of the question, understanding osmosis helps determine the direction of water movement in relation to the solute concentrations inside and outside the artificial cell.
Recommended video:
Guided course
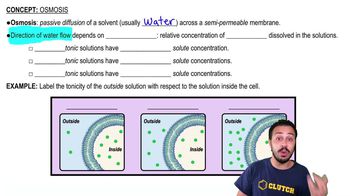
Osmosis
Tonicity
Tonicity describes the relative concentration of solutes in two solutions separated by a semipermeable membrane, influencing the movement of water. Solutions can be isotonic (equal solute concentration), hypotonic (lower solute concentration outside the cell), or hypertonic (higher solute concentration outside the cell). Identifying the tonicity of the external solution is essential for predicting whether the artificial cell will swell, shrink, or remain unchanged.
Recommended video:
Guided course

Environmental Tonicity Affects Cells
Related Practice
Textbook Question
2144
views
Textbook Question
Which of the following processes includes all the others? a. osmosis b. diffusion of a solute across a membrane c. passive transport d. transport of an ion down its electrochemical gradient
1329
views
Textbook Question
Based on Figure 7.18, which of these experimental treatments would increase the rate of sucrose transport into a plant cell? a. decreasing extracellular sucrose concentration b. decreasing extracellular pH c. decreasing cytoplasmic pH d. adding a substance that makes the membrane more permeable to hydrogen ions
1439
views
