Here are the essential concepts you must grasp in order to answer the question correctly.
Properties of Water
Water is known for its unique properties, including being a polar molecule, which allows it to dissolve many substances, making it an excellent solvent. This property is crucial for biological processes, as it facilitates the transport of nutrients and waste in organisms.
Recommended video:
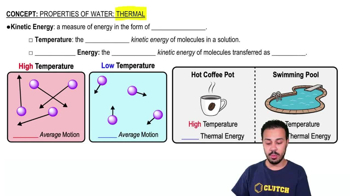
Properties of Water- Thermal
Role of Water in Chemical Reactions
Water plays a vital role in biochemical reactions, often acting as a medium where reactions occur. It participates in hydrolysis and condensation reactions, which are essential for breaking down and synthesizing biomolecules, respectively.
Recommended video:
Molecular Structure of Water
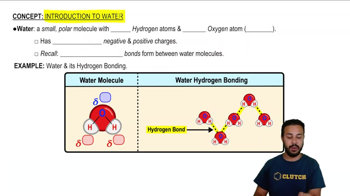
The molecular structure of water consists of two hydrogen atoms covalently bonded to one oxygen atom, forming a bent shape. This arrangement leads to hydrogen bonding between water molecules, contributing to its high specific heat, surface tension, and solvent capabilities.
Recommended video:
 Verified step by step guidance
Verified step by step guidance