Functional groups are specific groups of atoms within molecules that are responsible for the chemical reactions of those molecules. They are commonly found in biomolecules and extend off the carbon backbone, which is often represented by squiggly lines in structural formulas. Understanding these functional groups is essential as they play a crucial role in the structure and function of various biological molecules.
In biology, there are seven primary functional groups that are important to know:
The methyl group consists of a carbon atom bonded to three hydrogen atoms, typically found in lipids. This group is represented as -CH3.
The hydroxyl group features an oxygen atom bonded to a hydrogen atom, represented as -OH. This group is prevalent in carbohydrates and contributes to the solubility of organic compounds in water.
The carbonyl group is characterized by a carbon atom double-bonded to an oxygen atom, represented as >C=O. This group is commonly found in sugars and other biomolecules.
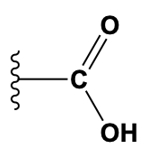
The carboxyl group combines both a carbonyl and a hydroxyl group, represented as -COOH. It is acidic and is found in amino acids and fatty acids, playing a significant role in metabolic processes.
The amino group contains a nitrogen atom bonded to two hydrogen atoms, represented as -NH2. This group is essential in amino acids, the building blocks of proteins.
The phosphate group consists of a phosphorus atom bonded to four oxygen atoms, typically represented as -PO43-. It is crucial in energy transfer through ATP and is a key component of nucleic acids.
Lastly, the sulfhydryl group contains a sulfur atom bonded to a hydrogen atom, represented as -SH. This group is important in the structure of proteins, particularly in forming disulfide bonds that stabilize protein structure.
Familiarity with these functional groups will aid in recognizing their roles in various biomolecules and understanding their chemical behavior. As you progress in your studies, you may need to memorize their structures and functions, as they will frequently appear in discussions of biological processes and molecular interactions.