In this video, we're going to talk about some strategic ways to use SDS PAGE. So as you guys already know, SDS PAGE separates proteins only based on their mass. Unlike native PAGE, SDS PAGE actually allows for the separation of protein subunits. However, it only allows the separation of protein subunits that are not covalently linked together. If we're interested in separating protein subunits that are covalently linked, then we need a way to break those covalent bonds that link them together. That's when a molecule such as beta-mercaptoethanol can come into play. Recall from our previous lessons on the Anfinsen experiment and denaturation that beta-mercaptoethanol can be abbreviated as βME, and it's specifically used to cleave covalent disulfide bonds.
In our example below, we're going to talk about how we can strategically use beta-mercaptoethanol with SDS PAGE to obtain information about a protein structure. Also, in the example, we're going to label each of the protein bands that we see throughout our gel with the appropriate protein subunits. Notice that on the left over here, what we have is a protein with a quaternary structure, because we have these different subunits: subunit a, subunit b, subunit c, and subunit d. Subunit b and subunit d associate with subunit a via only non-covalent interactions. However, subunit a and subunit c interact with each other via non-covalent interactions, but also a covalent disulfide bond that's present here. That's going to be very important to keep in mind as we analyze our gels on the right.
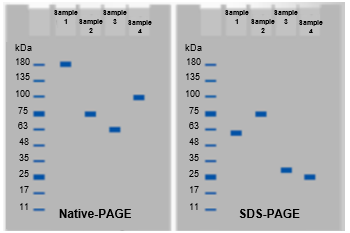
Notice that the first gel that we have is the native PAGE gel. With native PAGE, recall that the proteins retain their native shapes, their native charges, and their native masses. Essentially, the protein retains all of its native properties. What you'll notice is that our entire protein is going to remain intact, and this single protein band that we see here represents all of our protein subunits. It has subunit a, subunits b, c, and subunit d. Remember that with native PAGE, there are three factors that influence the migration of the protein through the gel. Again, those are the shape, the mass, and the charge of the proteins, and only proteins with native charges will migrate through the gel with native PAGE.
Now, with SDS PAGE, we are adding the molecule SDS, which is sodium dodecyl sulfate. We know that SDS is a highly non-polar detergent with a negative charge. SDS is going to denature all of our subunits in our protein. It's important to keep in mind that SDS, even though it denatures the proteins, does not cleave disulfide bonds. So the disulfide bond is going to remain intact, and that means that subunit a is going to remain connected to subunit c and they are going to travel together through the gel and appear as a single band, whereas subunit b and subunit d, which do not have disulfide bonds, are going to separate and appear as their own bands because they are different sizes. With SDS PAGE, proteins are separated only based on their size. Because subunit a is the largest subunit and it's connected to subunit c, it's going to be the largest protein band on our SDS PAGE gel, and the largest protein band travels the slowest.
This protein band represents subunit a as well as subunit c because those two are connected via our disulfide bond. The next protein band that we see would be the next largest subunit, which is subunit b, because subunit b is larger than subunit d. That means that the next one is going to be subunit b, and our smallest protein band at the very bottom that travels the fastest is going to be our smallest subunit, which is subunit d.
Now, if we're interested in strategically using beta-mercaptoethanol with SDS PAGE, it means that beta-mercaptoethanol is going to cleave this disulfide bond, which means that once this disulfide bond has been cleaved in the presence of SDS, these two subunits are finally going to be separated from one another. What you'll notice is that the largest subunit is going to travel the slowest, and so the largest subunit here would be subunit a. Then the next largest subunit would be the one that would be next in line, which would be subunit b, which, notice, stays in the same exact position. Subunit b has not changed its position in this gel. Now we have an additional band here that wasn't present, and this is the band for subunit c since that's the next largest behind subunit b. Subunit c is next, and then, of course, the smallest subunit is going to be subunit d here, which also has not changed. What you can see here is that this subunit a at the top has split and it formed these two bands that we see here. This is what these dotted red lines represent: this protein band that we see here is actually representing subunit a and subunit c that were covalently bound by a disulfide bond previous to breaking it with beta-mercaptoethanol.