In this video, we're going to introduce cyclic monosaccharides. Now, before we get started, it's important to note that up until this point in our course, we've mainly been focusing on the open or linear forms of monosaccharides. However, in biological solutions, most carbohydrates that have at least 5 carbon atoms exist in their cyclic forms, and so cyclic monosaccharides are very important when it comes to biochemistry. Now monosaccharides can actually cyclize to form many different types of rings, including 3, 4, 5, 6, and sometimes even 7-membered rings. However, the most common rings are 5 and 6-membered rings, and that's because 5 and 6-membered rings are actually the most stable rings. Now monosaccharides that have a 5-membered ring are called furanoses, and monosaccharides that have a 6-membered ring are called pyranoses. Now, of course, we know that the ending ose indicates a sugar, but why the prefix furan and why the prefix pyran? Well, it's because furanoses resemble the structure of furan. And so if we take a look at our image down below over here on the left-hand side, you can see that we're showing you the furan ring and the pyran ring structure down below. And so you can see that the furan ring is indeed a 5-membered ring. And so you can think the f in furan is for the 5-membered ring. And then, of course, the pyran ring is going to be a 6-membered ring. Now one thing to not get tripped up on is that the furan ring, even though it is a 5-membered ring, it does not have 5 carbons in its ring. Notice that it only has 4 carbon atoms in its ring, and the 5th member is actually an oxygen atom. And the same thing applies for the pyran ring. It is a 6-membered ring but does not have 6 carbons in its ring. Instead, it only has 5 carbons in its ring, and the 6th member is an oxygen atom. And the same thing applies to furanoses and pyranoses. And so notice over here, we're showing you the formation of a furanose. And down below, we're showing the formation of a pyranose. And so you can see that we're taking up at the top here the linear form of D-fructose. And then we've got this muscle man here bending the D-glucose molecule and really the muscle man is just here to help you guys better visualize the bending of the molecule. But the muscle man does not represent an enzyme as we'll find out later in our course. These cyclic structures will actually form without an enzyme. And so, here we can see that the C_5 hydroxyl group is going to interact with the ketone here on the C_2 carbon. And we'll talk more about this detailed reaction a little later in our course, but these two groups are going to react to create this furanose over here. And so you can see that the 5-membered ring resembles the 5-membered furanose ring, even though the double bonds aren't present over here, that's okay. You can still see the resemblance and that's why we call this D-fructofuranose. Now, moving on to the image down below, notice that we're starting with the linear form of the sugar D-glucose. And again, we've got this muscle man here just to help you guys better visualize the bending of the molecule and the muscle man does not represent an enzyme. However, here, notice that the C_5 hydroxyl group is interacting with the aldehyde group on the C_1 carbon instead of interacting with the ketone on the C_2. And so really that's the main difference that generates this pyranose over here. And again, you can see that this 6-membered ring resembles the 6-membered ring of the pyran even though the double bonds here and here are not present, over here. That's okay because we can still see the resemblance. And so this is going to be D-glucopyranose. And so, really, this here concludes our introduction to furanoses and pyranoses, and we'll continue to talk more about cyclic monosaccharides as we move forward in our course. So I'll see you guys in our next video
- 1. Introduction to Biochemistry4h 34m
- What is Biochemistry?5m
- Characteristics of Life12m
- Abiogenesis13m
- Nucleic Acids16m
- Proteins12m
- Carbohydrates8m
- Lipids10m
- Taxonomy10m
- Cell Organelles12m
- Endosymbiotic Theory11m
- Central Dogma22m
- Functional Groups15m
- Chemical Bonds13m
- Organic Chemistry31m
- Entropy17m
- Second Law of Thermodynamics11m
- Equilibrium Constant10m
- Gibbs Free Energy37m
- 2. Water3h 23m
- 3. Amino Acids8h 10m
- Amino Acid Groups8m
- Amino Acid Three Letter Code13m
- Amino Acid One Letter Code37m
- Amino Acid Configuration20m
- Essential Amino Acids14m
- Nonpolar Amino Acids21m
- Aromatic Amino Acids14m
- Polar Amino Acids16m
- Charged Amino Acids40m
- How to Memorize Amino Acids1h 7m
- Zwitterion33m
- Non-Ionizable Vs. Ionizable R-Groups11m
- Isoelectric Point10m
- Isoelectric Point of Amino Acids with Ionizable R-Groups51m
- Titrations of Amino Acids with Non-Ionizable R-Groups44m
- Titrations of Amino Acids with Ionizable R-Groups38m
- Amino Acids and Henderson-Hasselbalch44m
- 4. Protein Structure10h 4m
- Peptide Bond18m
- Primary Structure of Protein31m
- Altering Primary Protein Structure15m
- Drawing a Peptide44m
- Determining Net Charge of a Peptide42m
- Isoelectric Point of a Peptide37m
- Approximating Protein Mass7m
- Peptide Group22m
- Ramachandran Plot26m
- Atypical Ramachandran Plots12m
- Alpha Helix15m
- Alpha Helix Pitch and Rise20m
- Alpha Helix Hydrogen Bonding24m
- Alpha Helix Disruption23m
- Beta Strand12m
- Beta Sheet12m
- Antiparallel and Parallel Beta Sheets39m
- Beta Turns26m
- Tertiary Structure of Protein16m
- Protein Motifs and Domains23m
- Denaturation14m
- Anfinsen Experiment20m
- Protein Folding34m
- Chaperone Proteins19m
- Prions4m
- Quaternary Structure15m
- Simple Vs. Conjugated Proteins10m
- Fibrous and Globular Proteins11m
- 5. Protein Techniques14h 5m
- Protein Purification7m
- Protein Extraction5m
- Differential Centrifugation15m
- Salting Out18m
- Dialysis9m
- Column Chromatography11m
- Ion-Exchange Chromatography35m
- Anion-Exchange Chromatography38m
- Size Exclusion Chromatography28m
- Affinity Chromatography16m
- Specific Activity16m
- HPLC29m
- Spectrophotometry51m
- Native Gel Electrophoresis23m
- SDS-PAGE34m
- SDS-PAGE Strategies16m
- Isoelectric Focusing17m
- 2D-Electrophoresis23m
- Diagonal Electrophoresis29m
- Mass Spectrometry12m
- Mass Spectrum47m
- Tandem Mass Spectrometry16m
- Peptide Mass Fingerprinting16m
- Overview of Direct Protein Sequencing30m
- Amino Acid Hydrolysis10m
- FDNB26m
- Chemical Cleavage of Bonds29m
- Peptidases1h 6m
- Edman Degradation30m
- Edman Degradation Sequenator and Sequencing Data Analysis4m
- Edman Degradation Reaction Efficiency20m
- Ordering Cleaved Fragments21m
- Strategy for Ordering Cleaved Fragments58m
- Indirect Protein Sequencing Via Geneomic Analyses24m
- 6. Enzymes and Enzyme Kinetics13h 38m
- Enzymes24m
- Enzyme-Substrate Complex17m
- Lock and Key Vs. Induced Fit Models23m
- Optimal Enzyme Conditions9m
- Activation Energy24m
- Types of Enzymes41m
- Cofactor15m
- Catalysis19m
- Electrostatic and Metal Ion Catalysis11m
- Covalent Catalysis18m
- Reaction Rate10m
- Enzyme Kinetics24m
- Rate Constants and Rate Law35m
- Reaction Orders52m
- Rate Constant Units11m
- Initial Velocity31m
- Vmax Enzyme27m
- Km Enzyme42m
- Steady-State Conditions25m
- Michaelis-Menten Assumptions18m
- Michaelis-Menten Equation52m
- Lineweaver-Burk Plot43m
- Michaelis-Menten vs. Lineweaver-Burk Plots20m
- Shifting Lineweaver-Burk Plots37m
- Calculating Vmax40m
- Calculating Km31m
- Kcat46m
- Specificity Constant1h 1m
- 7. Enzyme Inhibition and Regulation 8h 42m
- Enzyme Inhibition13m
- Irreversible Inhibition12m
- Reversible Inhibition9m
- Inhibition Constant26m
- Degree of Inhibition15m
- Apparent Km and Vmax29m
- Inhibition Effects on Reaction Rate10m
- Competitive Inhibition52m
- Uncompetitive Inhibition33m
- Mixed Inhibition40m
- Noncompetitive Inhibition26m
- Recap of Reversible Inhibition37m
- Allosteric Regulation7m
- Allosteric Kinetics17m
- Allosteric Enzyme Conformations33m
- Allosteric Effectors18m
- Concerted (MWC) Model25m
- Sequential (KNF) Model20m
- Negative Feedback13m
- Positive Feedback15m
- Post Translational Modification14m
- Ubiquitination19m
- Phosphorylation16m
- Zymogens13m
- 8. Protein Function 9h 41m
- Introduction to Protein-Ligand Interactions15m
- Protein-Ligand Equilibrium Constants22m
- Protein-Ligand Fractional Saturation32m
- Myoglobin vs. Hemoglobin27m
- Heme Prosthetic Group31m
- Hemoglobin Cooperativity23m
- Hill Equation21m
- Hill Plot42m
- Hemoglobin Binding in Tissues & Lungs31m
- Hemoglobin Carbonation & Protonation19m
- Bohr Effect23m
- BPG Regulation of Hemoglobin24m
- Fetal Hemoglobin6m
- Sickle Cell Anemia24m
- Chymotrypsin18m
- Chymotrypsin's Catalytic Mechanism38m
- Glycogen Phosphorylase21m
- Liver vs Muscle Glycogen Phosphorylase21m
- Antibody35m
- ELISA15m
- Motor Proteins14m
- Skeletal Muscle Anatomy22m
- Skeletal Muscle Contraction45m
- 9. Carbohydrates7h 49m
- Carbohydrates19m
- Monosaccharides15m
- Stereochemistry of Monosaccharides33m
- Monosaccharide Configurations32m
- Cyclic Monosaccharides20m
- Hemiacetal vs. Hemiketal19m
- Anomer14m
- Mutarotation13m
- Pyranose Conformations23m
- Common Monosaccharides33m
- Derivatives of Monosaccharides21m
- Reducing Sugars21m
- Reducing Sugars Tests19m
- Glycosidic Bond48m
- Disaccharides40m
- Glycoconjugates12m
- Polysaccharide7m
- Cellulose7m
- Chitin8m
- Peptidoglycan12m
- Starch13m
- Glycogen14m
- Lectins16m
- 10. Lipids5h 49m
- Lipids15m
- Fatty Acids30m
- Fatty Acid Nomenclature11m
- Omega-3 Fatty Acids12m
- Triacylglycerols11m
- Glycerophospholipids24m
- Sphingolipids13m
- Sphingophospholipids8m
- Sphingoglycolipids12m
- Sphingolipid Recap22m
- Waxes5m
- Eicosanoids19m
- Isoprenoids9m
- Steroids14m
- Steroid Hormones11m
- Lipid Vitamins19m
- Comprehensive Final Lipid Map13m
- Biological Membranes16m
- Physical Properties of Biological Membranes18m
- Types of Membrane Proteins8m
- Integral Membrane Proteins16m
- Peripheral Membrane Proteins12m
- Lipid-Linked Membrane Proteins21m
- 11. Biological Membranes and Transport 6h 37m
- Biological Membrane Transport21m
- Passive vs. Active Transport18m
- Passive Membrane Transport21m
- Facilitated Diffusion8m
- Erythrocyte Facilitated Transporter Models30m
- Membrane Transport of Ions29m
- Primary Active Membrane Transport15m
- Sodium-Potassium Ion Pump20m
- SERCA: Calcium Ion Pump10m
- ABC Transporters12m
- Secondary Active Membrane Transport12m
- Glucose Active Symporter Model19m
- Endocytosis & Exocytosis18m
- Neurotransmitter Release23m
- Summary of Membrane Transport21m
- Thermodynamics of Membrane Diffusion: Uncharged Molecule51m
- Thermodynamics of Membrane Diffusion: Charged Ion1h 1m
- 12. Biosignaling9h 45m
- Introduction to Biosignaling44m
- G protein-Coupled Receptors32m
- Stimulatory Adenylate Cyclase GPCR Signaling42m
- cAMP & PKA28m
- Inhibitory Adenylate Cyclase GPCR Signaling29m
- Drugs & Toxins Affecting GPCR Signaling20m
- Recap of Adenylate Cyclase GPCR Signaling5m
- Phosphoinositide GPCR Signaling58m
- PSP Secondary Messengers & PKC27m
- Recap of Phosphoinositide Signaling7m
- Receptor Tyrosine Kinases26m
- Insulin28m
- Insulin Receptor23m
- Insulin Signaling on Glucose Metabolism57m
- Recap Of Insulin Signaling in Glucose Metabolism6m
- Insulin Signaling as a Growth Factor1h 1m
- Recap of Insulin Signaling As A Growth Factor9m
- Recap of Insulin Signaling1m
- Jak-Stat Signaling25m
- Lipid Hormone Signaling15m
- Summary of Biosignaling13m
- Signaling Defects & Cancer20m
- Review 1: Nucleic Acids, Lipids, & Membranes2h 47m
- Nucleic Acids 19m
- Nucleic Acids 211m
- Nucleic Acids 34m
- Nucleic Acids 44m
- DNA Sequencing 19m
- DNA Sequencing 211m
- Lipids 111m
- Lipids 24m
- Membrane Structure 110m
- Membrane Structure 29m
- Membrane Transport 18m
- Membrane Transport 24m
- Membrane Transport 36m
- Practice - Nucleic Acids 111m
- Practice - Nucleic Acids 23m
- Practice - Nucleic Acids 39m
- Lipids11m
- Practice - Membrane Structure 17m
- Practice - Membrane Structure 25m
- Practice - Membrane Transport 16m
- Practice - Membrane Transport 26m
- Review 2: Biosignaling, Glycolysis, Gluconeogenesis, & PP-Pathway3h 12m
- Biosignaling 19m
- Biosignaling 219m
- Biosignaling 311m
- Biosignaling 49m
- Glycolysis 17m
- Glycolysis 27m
- Glycolysis 38m
- Glycolysis 410m
- Fermentation6m
- Gluconeogenesis 18m
- Gluconeogenesis 27m
- Pentose Phosphate Pathway15m
- Practice - Biosignaling13m
- Practice - Bioenergetics 110m
- Practice - Bioenergetics 216m
- Practice - Glycolysis 111m
- Practice - Glycolysis 27m
- Practice - Gluconeogenesis5m
- Practice - Pentose Phosphate Path6m
- Review 3: Pyruvate & Fatty Acid Oxidation, Citric Acid Cycle, & Glycogen Metabolism2h 26m
- Pyruvate Oxidation9m
- Citric Acid Cycle 114m
- Citric Acid Cycle 27m
- Citric Acid Cycle 37m
- Citric Acid Cycle 411m
- Metabolic Regulation 18m
- Metabolic Regulation 213m
- Glycogen Metabolism 16m
- Glycogen Metabolism 28m
- Fatty Acid Oxidation 111m
- Fatty Acid Oxidation 28m
- Citric Acid Cycle Practice 17m
- Citric Acid Cycle Practice 26m
- Citric Acid Cycle Practice 32m
- Glucose and Glycogen Regulation Practice 14m
- Glucose and Glycogen Regulation Practice 26m
- Fatty Acid Oxidation Practice 14m
- Fatty Acid Oxidation Practice 27m
- Review 4: Amino Acid Oxidation, Oxidative Phosphorylation, & Photophosphorylation1h 48m
- Amino Acid Oxidation 15m
- Amino Acid Oxidation 211m
- Oxidative Phosphorylation 18m
- Oxidative Phosphorylation 210m
- Oxidative Phosphorylation 310m
- Oxidative Phosphorylation 47m
- Photophosphorylation 15m
- Photophosphorylation 29m
- Photophosphorylation 310m
- Practice: Amino Acid Oxidation 12m
- Practice: Amino Acid Oxidation 22m
- Practice: Oxidative Phosphorylation 15m
- Practice: Oxidative Phosphorylation 24m
- Practice: Oxidative Phosphorylation 35m
- Practice: Photophosphorylation 15m
- Practice: Photophosphorylation 21m
Cyclic Monosaccharides: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AICyclic monosaccharides, such as furanoses and pyranoses, are crucial in biochemistry, predominantly existing in 5 or 6 membered rings due to their stability. The anomeric carbon, attached to two oxygen atoms, is pivotal for numbering these structures. Haworth projections depict these cyclic forms, emphasizing 3D geometry with thicker lines for bonds closer to the viewer. Converting Fischer projections to Haworth involves understanding that left-pointing groups become upward and right-pointing groups become downward. This knowledge is essential for grasping carbohydrate structure and function in biological systems.
Cyclic Monosaccharides
Video transcript
Cyclic Monosaccharides
Video transcript
In this video, we're going to introduce a relatively easy way to convert Fisher projections into Haworth projections. And so all we need to remember is that chemical groups that are pointing to the left of a Fischer projection are going to be pointing upwards in a Haworth projection. And then of course, chemical groups that are pointing right of a Fisher projection are going to be pointing downwards in a Haworth projection. And so we can see over here with this Fisher projection, all of the left pointing groups are going to end up pointing upwards in the Haworth projection, whereas all of the right pointing groups are going to end up pointing downwards in the Haworth projection. If you remember the term uplifting, then you'll be able to associate left pointing groups of the Fisher projection with upwards pointing groups in the Haworth projection. And then if you remember the term downright, you'll be able to associate right pointing groups in the Fisher projection with downward pointing groups in the Haworth projection.
So let's go ahead and apply what we've learned here in our lesson to the D-glucose molecule that we see on the right. Notice that we have these very specific hydroxyl groups highlighted here, and we want to know if these hydroxyl groups are going to be pointing upwards or downwards in the Haworth projection. Looking at carbon number 2 right here, we can see that the hydroxyl group is pointing to the right. If it's pointing to the right, it's going to be pointing downwards in the Haworth projection. So with carbon number 2 here, we expect the hydroxyl group to be pointing downwards. So we can go ahead and put in an "OH" right here, and of course, this means that the hydrogen atom going over here is going to be pointing upwards since it's pointing to the left.
Now moving on to carbon number 3, notice that carbon number 3's hydroxyl group is pointing to the left. And of course, if you remember left, uplifting, you know that if it's pointing to the left, it's going to end up pointing upwards in the Haworth projection. So we know that the hydroxyl group is going to be pointing upwards. And, of course, that means that the hydrogen atom over here, which is pointing to the right, is going to be going downwards. So we can put the hydrogen here.
Last but not least, looking at carbon number 4, you can see that its hydroxyl group is also pointing to the right, which means that in the Haworth projection, it's going to be pointing downwards. And so, we can go ahead and put in the hydroxyl group going downwards, and of course, this means that the hydrogen atom, which is pointing left, is going to be pointing upwards in the Haworth projection.
By remembering uplifting, and downfall, you'll be able to easily convert Fisher projections into Haworth projections. That concludes this part of the lesson, and again, we'll be able to apply the concepts that we've learned here as we move forward in our course. I'll see you guys in our next video.
Cyclic Monosaccharides
Video transcript
So now that we've introduced cyclic monosaccharides like furanoses and pyranoses, in this video we're going to talk about how cyclic monosaccharide structures are commonly depicted with Haworth projections. And so if we take a look down below at our image, notice on the left-hand side over here, we have an open chain or a linear form of D-glucose. And of course, we know that this structure here is an example of a Fischer projection. Now over here, notice we have the cyclic form of D-glucose, shown as D-glucopyranose because it has a 6-membered ring. And so, what we're saying in this video is that cyclic monosaccharides are depicted with Haworth projection. So this is indeed a Haworth projection. And so what you'll notice is that in Haworth projections, these bonds towards the front are darker and thicker. And so the darker, thicker lines, you can imagine them as popping out of the page at us so that these bonds are closer to us as the readers. Also, notice that we have these lighter, thinner bonds that are in the back, and so these lighter, thinner lines, you can imagine them as going into the page, so that they are further away from us as the reader. And so really, the Haworth projection is supposed to bring a sense of 3-dimensionality, just like the Fischer projection is supposed to bring a sense of 3-dimensionality.
Now, also, just like there are standard Fischer projections where the carbonyl group is towards the top and the longest carbon chain is vertical, there are also standard Haworth projections. And so the standard Haworth projections will have what is known as the anomeric carbon, on the right side of the structure, and we'll define the anomeric carbon later in our course. However, the anomeric carbon in this structure is this carbon right here. So the anomeric carbon is on the right-hand side. And, with standard Haworth projections, the highest numbered carbon, so numbering these carbons, is going to be pointing upwards. And so you can see that the highest numbered carbon, these red numbers show, the numbering of the carbons, and you can see that the highest numbered carbon, which is carbon 6 here, is pointing in an upwards fashion. And so, really this is the standard Haworth projection. But again, we'll get more practice utilizing and recognizing standard Haworth projections as we move along through our course. But really, the main takeaway here in this lesson is that Haworth projections are used for cyclic monosaccharides whereas Fischer projections are used for linear monosaccharides.
Now, as we move forward, it's also important to keep in mind that Haworth projections can actually sometimes be a little bit misleading, and that's because cyclic monosaccharides are actually not planar. So when we look at this Haworth projection, you might assume that all of these carbons that are being shown here, all of the members of the ring, I should say, all of them, you might assume, are in the same plane because they appear to be flat in this Haworth projection. But that's a little bit misleading, as I said because the cyclic monosaccharide here is not planar, and that's because recall that every carbon atom is going to have tetrahedral geometry. And so later in our course, we're going to talk about other ways to display cyclic monosaccharides. And so this here concludes our introduction to Haworth projections and again we'll be able to apply these concepts as we move forward in our course. So I'll see you guys in our next video.
Cyclic Monosaccharides
Video transcript
So recall that in one of our older lesson videos, we had talked about how to assign numbers to the carbon atoms of linear monosaccharides. But in this video, we're going to talk about how to assign numbers to the carbon atoms of cyclic monosaccharides, which is actually going to be a little bit different. The carbon atoms in cyclic monosaccharides are going to be numbered based on the positioning of what's known as the anomeric carbon. But what in the world is the anomeric carbon? Well, the anomeric carbon is going to be the only carbon atom that is covalently attached to two oxygen atoms directly.
And so, if we take a look down below at our cyclic sugar molecule, notice that the anomeric carbon is highlighted with this green background right here. And so if we take a closer look at this anomeric carbon, notice that it is directly bound to two oxygen atoms. One oxygen atom is the ring oxygen, and the other oxygen atom is the oxygen of the hydroxyl group. If you take a look at all of the other carbon atoms, none of these carbon atoms are covalently attached to two oxygen atoms like the anomeric carbon is, and so that makes it pretty easy to identify the anomeric carbon.
Now, when it comes to numbering the carbon atoms of cyclic monosaccharides, we need to prioritize making the anomeric carbon the lowest possible number. We want to assign the anomeric carbon the lowest possible number. When we take a look at the anomeric carbon over here, the lowest possible number that we can give it is carbon number 1. And then, of course, we can start to number the carbons sequentially as they are connected. So this carbon atom would be carbon number 2. This carbon atom would be carbon number 3. This carbon atom is carbon number 4. This is carbon number 5 and then carbon atom number 6 is up here. This is the proper numbering of the carbon atoms in this glucose molecule in our example.
As we move forward in our course, we'll be able to get some practice identifying the anomeric carbon and we'll get some practice numbering the carbon atoms of cyclic monosaccharides. So I'll see you guys in our next video.
Below is the structure for a cyclic D-monosaccharide. Which labeled carbon is the anomeric carbon?
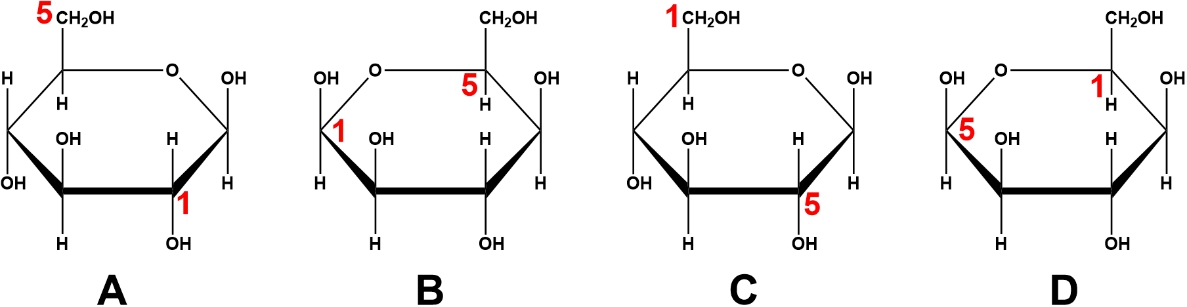
Which image represents the proper convention for carbon numbering of cyclic sugars?
Here’s what students ask on this topic:
What are cyclic monosaccharides and why are they important in biochemistry?
Cyclic monosaccharides are forms of monosaccharides (simple sugars) that exist in ring structures rather than linear forms. They are crucial in biochemistry because most carbohydrates with at least five carbon atoms predominantly exist in their cyclic forms in biological solutions. These cyclic forms, such as furanoses (5-membered rings) and pyranoses (6-membered rings), are more stable and play essential roles in various biological processes, including energy storage and structural functions. Understanding cyclic monosaccharides is fundamental for grasping carbohydrate structure and function in biological systems.
 Created using AI
Created using AIHow do you convert Fischer projections to Haworth projections?
To convert Fischer projections to Haworth projections, remember that groups pointing to the left in a Fischer projection will point upwards in a Haworth projection, and groups pointing to the right will point downwards. For example, in D-glucose, the hydroxyl group on carbon 2 points to the right in the Fischer projection, so it will point downwards in the Haworth projection. Similarly, the hydroxyl group on carbon 3 points to the left in the Fischer projection, so it will point upwards in the Haworth projection. This method helps visualize the 3D structure of cyclic monosaccharides.
 Created using AI
Created using AIWhat is the anomeric carbon in cyclic monosaccharides?
The anomeric carbon in cyclic monosaccharides is the carbon atom that is covalently attached to two oxygen atoms. It is a key feature for numbering the carbon atoms in these structures. In a Haworth projection, the anomeric carbon is typically found on the right side of the ring. For example, in glucose, the anomeric carbon is carbon 1, which is bonded to the ring oxygen and the hydroxyl group. Identifying the anomeric carbon is essential for understanding the structure and reactivity of cyclic monosaccharides.
 Created using AI
Created using AIWhat are Haworth projections and how are they used to depict cyclic monosaccharides?
Haworth projections are a way to represent the 3D structure of cyclic monosaccharides in a two-dimensional format. In these projections, bonds closer to the viewer are depicted with thicker lines, while bonds further away are shown with thinner lines. This method helps visualize the ring structure and spatial arrangement of atoms. For example, in D-glucopyranose, the Haworth projection shows the 6-membered ring with the anomeric carbon on the right and the highest numbered carbon pointing upwards. Haworth projections are essential for understanding the geometry and reactivity of cyclic sugars.
 Created using AI
Created using AIWhy are 5 and 6 membered rings the most common forms of cyclic monosaccharides?
5 and 6 membered rings are the most common forms of cyclic monosaccharides because they are the most stable. This stability arises from the fact that these ring sizes minimize angle strain and torsional strain, making them energetically favorable. For example, furanoses have 5-membered rings, and pyranoses have 6-membered rings. These stable ring structures are crucial for the biological functions of carbohydrates, such as energy storage and structural roles in cells.
 Created using AI
Created using AI