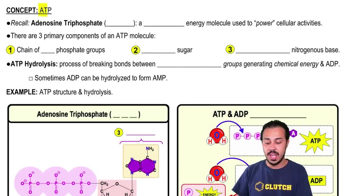
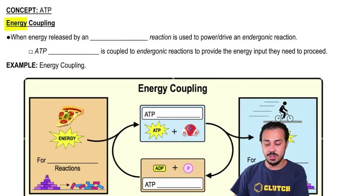
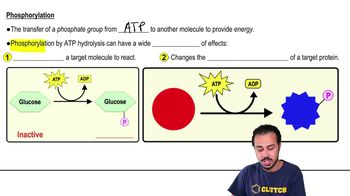
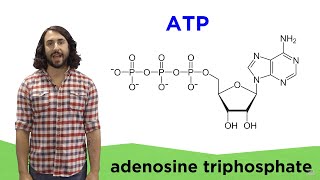
3. Energy & Cell Processes
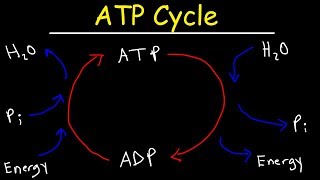
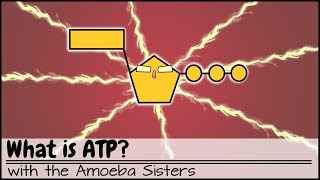
ATP
3. Energy & Cell Processes
ATP
Additional 6 creators.
Learn with other creators
Showing 9 of 9 videos
Practice this topic
- Multiple Choice
Which of the following statements is true?
8687views77rank1comments - Multiple Choice
How does ATP participate in energy-coupling reactions?
5363views49rank - Multiple ChoiceWhat is ATP's importance in the cell?3376views1rank
- Multiple ChoiceIn general, the hydrolysis of ATP drives cellular work by __________.2405views
- Textbook QuestionThe synthesis of ATP from ADP anda. stores energy in a form that can drive cellular work.b. involves the hydrolysis of a phosphate bond.c. transfers a phosphate, priming a protein to do work.d. is an exergonic process.1802views
- Textbook QuestionIn Figure 8.10, the energetic coupling of substrate phosphorylation and an endergonic reaction are shown. If the hydrolysis of ATP releases 7.3 kcal of free energy, use the graph in this figure to estimate what you would expect the ∆G values to be for the uncoupled reaction and the two steps in the coupled reaction.1882views
- Textbook QuestionWhat are the main types of cellular work? How does ATP provide the energy for this work?2166views
- Textbook Question
Which of the following statements is/are true regarding ATP?
a. It is the body's primary source of chemical energy.
b. Its production is the main reason humans breathe oxygen.
c. It is produced using the energy from the oxidation of molecular fuels like glucose.
d. All of the above statements are true.
934views