Table of contents
- 1. Introduction to Anatomy & Physiology
- What is Anatomy & Physiology?
- Levels of Organization
- Variation in Anatomy & Physiology
- Introduction to Organ Systems
- Homeostasis
- Feedback Loops
- Feedback Loops: Negative Feedback
- Feedback Loops: Positive Feedback
- Anatomical Position
- Introduction to Directional Terms
- Directional Terms: Up and Down
- Directional Terms: Front and Back
- Directional Terms: Body Sides
- Directional Terms: Limbs
- Directional Terms: Depth Within the Body
- Introduction to Anatomical Terms for Body Regions
- Anatomical Terms for the Head and Neck
- Anatomical Terms for the Front of the Trunk
- Anatomical Terms for the Back
- Anatomical Terms for the Arm and Hand
- Anatomical Terms for the Leg and Foot
- Review- Using Anatomical Terms and Directions
- Abdominopelvic Quadrants and Regions
- Anatomical Planes & Sections
- Organization of the Body: Body Cavities
- Organization of the Body: Serous Membranes
- Organization of the Body: Serous Membrane Locations
- Organization of the Body: Thoracic Cavity
- Organization of the Body: Abdominopelvic Cavity
- 2. Cell Chemistry & Cell Components
- Atoms- Smallest Unit of Matter
- Isotopes
- Introduction to Chemical Bonding
- Covalent Bonds
- Noncovalent Bonds
- Ionic Bonding
- Hydrogen Bonding
- Introduction to Water
- Properties of Water- Cohesion and Adhesion
- Properties of Water- Density
- Properties of Water- Thermal
- Properties of Water- The Universal Solvent
- Acids and Bases
- pH Scale
- Carbon
- Functional Groups
- Introduction to Biomolecules
- Monomers & Polymers
- Carbohydrates
- Proteins
- Nucleic Acids
- Lipids
- Microscopes
- Prokaryotic & Eukaryotic Cells
- Introduction to Eukaryotic Organelles
- Endomembrane System: Protein Secretion
- Endomembrane System: Digestive Organelles
- Mitochondria & Chloroplasts
- Endosymbiotic Theory
- Introduction to the Cytoskeleton
- Cell Junctions
- Biological Membranes
- Types of Membrane Proteins
- Concentration Gradients and Diffusion
- Introduction to Membrane Transport
- Passive vs. Active Transport
- Osmosis
- Simple and Facilitated Diffusion
- Active Transport
- Endocytosis and Exocytosis
- 3. Energy & Cell Processes
- Introduction to Energy
- Laws of Thermodynamics
- Chemical Reactions
- ATP
- Enzymes
- Enzyme Activation Energy
- Enzyme Binding Factors
- Enzyme Inhibition
- Introduction to Metabolism
- Redox Reactions
- Introduction to Cellular Respiration
- Types of Phosphorylation
- Glycolysis
- Pyruvate Oxidation
- Krebs Cycle
- Electron Transport Chain
- Chemiosmosis
- Review of Aerobic Cellular Respiration
- Fermentation & Anaerobic Respiration
- Introduction to Cell Division
- Organization of DNA in the Cell
- Introduction to the Cell Cycle
- Interphase
- Phases of Mitosis
- Cytokinesis
- Cell Cycle Regulation
- Review of the Cell Cycle
- Cancer
- Introduction to DNA Replication
- DNA Repair
- Central Dogma
- Introduction to Transcription
- Steps of Transcription
- Genetic Code
- Introduction to Translation
- Steps of Translation
- Post-Translational Modification
- 4. Tissues & Histology
- Introduction to Tissues & Histology
- Introduction to Epithelial Tissue
- Characteristics of Epithelial Tissue
- Structural Naming of Epithelial Tissue
- Simple Epithelial Tissues
- Stratified Epithelial Tissues
- Identifying Types of Epithelial Tissue
- Glandular Epithelial Tissue
- Introduction to Connective Tissue
- Classes of Connective Tissue
- Introduction to Connective Tissue Proper
- Connective Tissue Proper: Loose Connective Tissue
- Connective Tissue Proper: Dense Connective Tissue
- Specialized Connective Tissue: Cartilage
- Specialized Connective Tissue: Bone
- Specialized Connective Tissue: Blood
- Introduction to Muscle Tissue
- Types of Muscle Tissue
- Introduction to Nervous Tissue
- Nervous Tissue: The Neuron
- 5. Integumentary System
- 6. Bones & Skeletal Tissue
- An Introduction to Bone and Skeletal Tissue
- Gross Anatomy of Bone: Compact and Spongy Bone
- Gross Anatomy of Bone: Periosteum and Endosteum
- Gross Anatomy of Bone: Bone Marrow
- Gross Anatomy of Bone: Short, Flat, and Irregular Bones
- Gross Anatomy of Bones - Structure of a Long Bone
- Microscopic Anatomy of Bones - Bone Matrix
- Microscopic Anatomy of Bones - Bone Cells
- Microscopic Anatomy of Bones - The Osteon
- Microscopic Anatomy of Bones - Trabeculae
- 7. The Skeletal System
- 8. Joints
- 9. Muscle Tissue
- 10. Muscles
- 11. Nervous Tissue and Nervous System
- 12. The Central Nervous System
- 13. The Peripheral Nervous System
- 14. The Autonomic Nervous System
- 15. The Special Senses
- 16. The Endocrine System
- 17. The Blood
- 18. The Heart
- 19. The Blood Vessels
- 20. The Lymphatic System
- 21. The Immune System
- Introduction to the Immune System
- Introduction to Innate Immunity
- Introduction to First-Line Defenses
- Physical Barriers in First-Line Defenses: Skin
- Physical Barriers in First-Line Defenses: Mucous Membrane
- First-Line Defenses: Chemical Barriers
- First-Line Defenses: Normal Microbiota
- Introduction to Cells of the Immune System
- Cells of the Immune System: Granulocytes
- Cells of the Immune System: Agranulocytes
- Introduction to Cell Communication
- Cell Communication: Surface Receptors & Adhesion Molecules
- Cell Communication: Cytokines
- Pattern Recognition Receptors (PRRs)
- Introduction to the Complement System
- Activation Pathways of the Complement System
- Effects of the Complement System
- Review of the Complement System
- Phagocytosis
- Introduction to Inflammation
- Steps of the Inflammatory Response
- Fever
- Interferon Response
- Review Map of Innate Immunity
- Introduction to Adaptive Immunity
- Antigens
- Introduction to T Lymphocytes
- Major Histocompatibility Complex Molecules
- Activation of T Lymphocytes
- Functions of T Lymphocytes
- Review of Cytotoxic vs Helper T Cells
- Introduction to B Lymphocytes
- Antibodies
- Classes of Antibodies
- Outcomes of Antibody Binding to Antigen
- T Dependent & T Independent Antigens
- Clonal Selection
- Antibody Class Switching
- Affinity Maturation
- Primary and Secondary Response of Adaptive Immunity
- Immune Tolerance
- Regulatory T Cells
- Natural Killer Cells
- Review of Adaptive Immunity
- 22. The Respiratory System
- 23. The Digestive System
- 24. Metabolism and Nutrition
- Essential Amino Acids
- Lipid Vitamins
- Cellular Respiration: Redox Reactions
- Introduction to Cellular Respiration
- Cellular Respiration: Types of Phosphorylation
- Cellular Respiration: Glycolysis
- Cellular Respiration: Pyruvate Oxidation
- Cellular Respiration: Krebs Cycle
- Cellular Respiration: Electron Transport Chain
- Cellular Respiration: Chemiosmosis
- Review of Aerobic Cellular Respiration
- Fermentation & Anaerobic Respiration
- Gluconeogenesis
- Fatty Acid Oxidation
- Amino Acid Oxidation
- 25. The Urinary System
- 26. Fluid and Electrolyte Balance, Acid Base Balance
- 27. The Reproductive System
- 28. Human Development
- 29. Heredity
2. Cell Chemistry & Cell Components
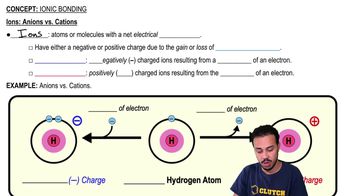
Ionic Bonding
2. Cell Chemistry & Cell Components
Ionic Bonding
+4
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
When atoms gain or lose electrons, they become negatively or positively charged. They are known as:
5873views62rank - Multiple Choice
Which of the following statements is true of ALL atoms that are anions?
6559views72rank - Multiple Choice
If oxygen has 9 electrons it will be a ______________________:
4890views79rank - Multiple Choice
An ionic bond is a bond in which:
4972views68rank - Textbook QuestionWhich statement is true of all atoms that are anions?a. The atom has more electrons than protons.b. The atom has more protons than electrons.c. The atom has fewer protons than does a neutral atom of the same element.d. The atom has more neutrons than protons.1881views