- 1. Chemical Measurements1h 50m
- 2. Tools of the Trade1h 17m
- 3. Experimental Error1h 52m
- 4 & 5. Statistics, Quality Assurance and Calibration Methods1h 57m
- 6. Chemical Equilibrium3h 41m
- 7. Activity and the Systematic Treatment of Equilibrium1h 0m
- 8. Monoprotic Acid-Base Equilibria1h 53m
- 9. Polyprotic Acid-Base Equilibria2h 17m
- 10. Acid-Base Titrations2h 37m
- 11. EDTA Titrations1h 34m
- 12. Advanced Topics in Equilibrium1h 16m
- 13. Fundamentals of Electrochemistry2h 19m
- 14. Electrodes and Potentiometry41m
- 15. Redox Titrations1h 14m
- 16. Electroanalytical Techniques57m
- 17. Fundamentals of Spectrophotometry50m
3. Experimental Error
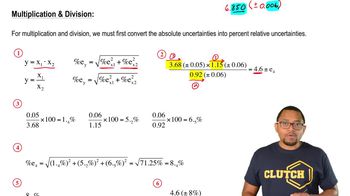
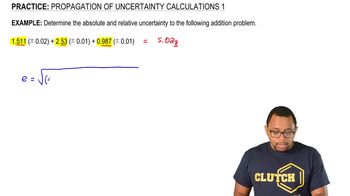
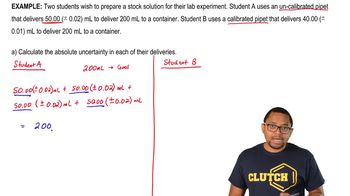
Uncertainty
Multiple Choice
I am making a 0.1 M KCl (molar mass 74.551) solution for an experiment. To measure the mass of the KCl, I will use an analytical balance that is only accurate to ± 0.01 g. I place a piece of paper on the balance and set the tare to read 0.00. I then put the KCl on the balance until it reads 6.79 g. What is the uncertainty in this mass?
A
0.010 g
B
0.014 g
C
0.0002 g
D
0.020 g

 1:48m
1:48mWatch next
Master Types of Uncertainty with a bite sized video explanation from Jules Bruno
Start learningRelated Videos
Related Practice